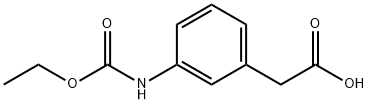
(3-Ethoxycarbonylaminophenyl)acetic acid synthesis
- Product Name:(3-Ethoxycarbonylaminophenyl)acetic acid
- CAS Number:741254-28-4
- Molecular formula:C11H13NO4
- Molecular Weight:223.23
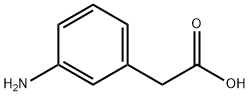
14338-36-4
168 suppliers
$15.00/1g
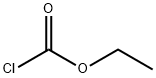
541-41-3
0 suppliers
$21.00/5g

741254-28-4
6 suppliers
$362.00/500mg
Yield:741254-28-4 96%
Reaction Conditions:
with sodium hydroxide in water at 0 - 20; for 16 h;
Steps:
4.1
To a solution of (3-AMINO-PHENYL)-ACETIC acid (10.6 g, 70.1 mmol) in 2 N NAOH (80 mL), cooled in an ice bath, was added ethyl chloroformate (8.37 g, 77.1 mmol) dropwise. The cooling bath was removed, and stirring was continued at room temperature for 16 hours. The mixture was then washed with diethyl ether, and the aqueous layer was separated, acidified with HCl, and the mixture was extracted with ethyl acetate. The ethyl acetate extracts were washed with brine and dried over NA2S04 to give (3-ETHOXYCARBONYLAMINO-PHENYL)-ACETIC acid, which crystallized upon standing. Yield 15.1 g (96 %). H NMR (400 MHz, CDC13) 8 11.35 (br s, 1H), 7.28-7. 20 (m, 3H), 6.96 (d, 1H), 6.68 (br s, 1H), 4.10 (q, 2H), 3.60 (s, 2H), 1.28 (t, 3H).
References:
WO2004/69245,2004,A1 Location in patent:Page/Page column 80-81