
3-(ethylaMino)-4-nitrobenzonitrile synthesis
- Product Name:3-(ethylaMino)-4-nitrobenzonitrile
- CAS Number:467235-08-1
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19
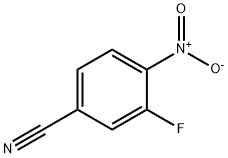
218632-01-0
165 suppliers
$7.00/1g
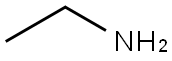
75-04-7
375 suppliers
$10.00/20mg

467235-08-1
8 suppliers
inquiry
Yield:467235-08-1 89%
Reaction Conditions:
with triethylamine in ethanol at 50; for 16 h;Inert atmosphere;
Steps:
24.1 Step 1: 3-(ethylamino)-4-nitrobenzonitrile
To a stirred mixture of 3-fluoro-4-nitrobenzonitrile (10.00 g, 60.20 mmol) and ethylamine (5.43 g, 120.40 mmol) in EtOH (90.00 mL) was added TEA (25.10 mL, 248.08 mmol) at room temperature under nitrogen atmosphere. The reaction mixture was stirred for 16 h at 50 °C. The resulting mixture was concentrated under reduced pressure. The residue was diluted with NaHCO3 (300 mL). The resulting mixture was extracted with EtOAc (3 x 200 mL). The combined organic layers were washed with brine (300 mL), dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated under reduced pressure. The residue was dried to offord 3-(ethylamino)-4-nitrobenzonitrile (11.5 g, 89%) which was used in the next step directly without further purification. H-NMR (400 MHz, Chloroform-d): δ 8.27 (d, J = 8.7 Hz, 1H), 7.98 (s, 1H), 7.17 (d, J = 1.7 Hz, 1H), 6.88 (dd, J = 8.7, 1.7 Hz, 1H), 3.41-3.38 (m, 2H), 1.43 (t, J = 7.2 Hz, 3H)
References:
WO2021/247969,2021,A1 Location in patent:Paragraph 00305-00307
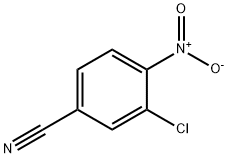
34662-29-8
53 suppliers
inquiry

467235-08-1
8 suppliers
inquiry
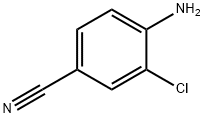
21803-75-8
194 suppliers
$6.00/1g

467235-08-1
8 suppliers
inquiry