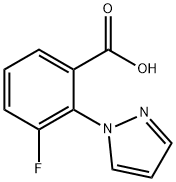
3-Fluoro-2-(1H-pyrazol-1-yl)benzoic acid synthesis
- Product Name:3-Fluoro-2-(1H-pyrazol-1-yl)benzoic acid
- CAS Number:1214622-53-3
- Molecular formula:C10H7FN2O2
- Molecular Weight:206.17
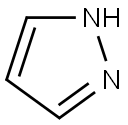
288-13-1
456 suppliers
$10.00/1g
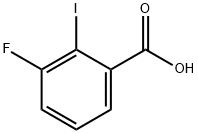
387-48-4
109 suppliers
$16.00/250mg

1214622-53-3
4 suppliers
$350.00/500mg
Yield:1214622-53-3 72%
Reaction Conditions:
Stage #1: NH-pyrazole;3-fluoro-2-iodobenzoic acidwith caesium carbonate;trans-N,N'-dimethyl-1,2-cyclohexyldiamine;copper(l) iodide in 1,4-dioxane;water at 100; for 1 h;
Stage #2: in water; pH=2;Acidic conditions;
Steps:
33
To a mixture of 3-fluoro-2-iodobenzoic acid (1 .4 g, 5.26 mmol), 1 H-pyrazole (0.72 g, 10.5 mmol), trans-N, N'-dimethyl-cyclohexane-1 ,2-diamine (0.17 mL, 1 .05 mmol), Cul (50.1 mg, 0.26 mmol), dioxane (50 mL) and water (0.028 mL) was added CS2CO3 (3.43 g, 10.5 mmol). The reaction mixture was heated to 100 °C for 1 h. The reaction mixture was cooled to ambient temperature then diluted with water. The aqueous layer was acidified to pH2 and extracted with EtOAc (30 mL) three times. The organic layers were combined, dried over Na2SO4, filtered and concentrated. Purification (FCC), (DCM to 10%MeOH/1 %HOAC/DCM) afforded the title compound as a colorless oil (790 mg, 72%). 1H NMR (400 MHz, CDCI3): 7.85 - 7.73 (m, 1 H), 7.54 - 7.44 (m, 1 H), 7.44 - 7.34 (m, 1 H), 6.55 (s, 1 H).
References:
WO2011/50200,2011,A1 Location in patent:Page/Page column 67