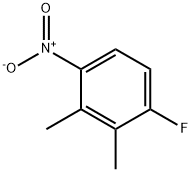
3-FLUORO-6-NITRO-O-XYLENE synthesis
- Product Name:3-FLUORO-6-NITRO-O-XYLENE
- CAS Number:1736-87-4
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
443-82-3

1736-87-4
Example 16: Synthesis of 2-(2,3-dimethyl-4-nitrophenylamino)-2-methylpropan-1-ol 1. fuming nitric acid (1.4 g, 20.3 mmol) was cooled to 0 °C and slowly added to acetic anhydride (2.89 g, 28.4 mmol). 2. the above solution was added dropwise to a cooled (0 °C) solution of 3-fluoro-1,2-dimethylbenzene (1.0 g, 8.1 mmol) in acetic anhydride (4 ml) within 10 min. 3. The reaction mixture was stirred at 0 °C for 25 min and then slowly poured into ice water. 4. The aqueous phase was extracted with EtOAc (3×) and the organic phases were combined and washed sequentially with saturated aqueous solution of dilute NaHCO3 and brine. 5. The organic phase was dried over anhydrous Na2SO4, concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (mobile phase: hexane) to obtain 2,3-dimethyl-4-fluoro-1-nitrobenzene 0.74 g (54% yield) as a yellow oil, which was crystallized after standing. 6. The above nitro compound (0.576 g, 3.4 mmol) was mixed with 2-amino-2-methylpropanol (0.61 g, 6.8 mmol) in a reaction tube, sealed and immersed in an oil bath at 160 °C for 5 days. 7. TLC monitoring (unfolding agent: hexane) showed raw material remaining. The reaction mixture was cooled and diluted with EtOAc. 8. Purification by fast silica gel column chromatography (dry on sample; eluent: hexane/EtOAc=6:4) recovered the feedstock 2,3-dimethyl-4-fluoro-1-nitrobenzene 0.34 g (59% recovery) and afforded the target product 2-(2,3-dimethyl-4-nitrophenylamino)-2-methylpropan-1-ol 0.20 g (61% yield based on recovered feedstock).

443-82-3
212 suppliers
$6.00/1g

1736-87-4
124 suppliers
$7.00/100mg
Yield: 54%
Reaction Conditions:
with nitric acid;acetic anhydride at 0; for 0.583333 h;
Steps:
16
Example 16 2- (2, 3-D6hyl-4-nitro-phenylamino} 2-methyl-propan-1-ol Fuming nitric acid (1.4 g, 20.3 mmol) was cooled to 0°C and acetic anhydride (2.89 g, 28.4 mmol) was added. This solution was added to a cold (0°C) solution of 3-fluoro-1, 2- dimethylbenzene (1.0 g, 8.1 mmol) in acetic anhydride (4 ml) over 10 min. The reaction mixture was stirred for 25 min, poured slowly over ice and the water solution extracted with EtOAc (x 3). The collected organic phase was washed with diluted saturated aqueous solution of NaHCO3 followed by brine before evaporation to dryness. The residue was flash purified on a silica gel column using hexane as a mobile phase to give 2, 3-dimethyl-4-fluoro-1-nitro-benzene 0.74 g (54%) as a yellow oil which crystallised upon standing. The fluoride (0.576 g, 3. 4 mmol) was mixed with 2-amino-2-methylpropanol (0.61 g, 6.8 mmol) in a tube, and the tube was sealed before immersing it into an oil bath and heating at 160°C for 5 days. TLC (Hexane) showed remaining starting material. The reaction mixture was cooled and diluted with EtOAc before purification by flash silica gel chromatography (dry application; 6: 4 hexane and EtOAc) to give 0.34 g (59% recovery) of the starting material 2, 3-dimethyl-4-fluoro-1-nitro-benzene and 0.20 g (61% based on recovered starting material) of the 2- (2, 3-dimethyl-4-nitro-phenylamino)-2-methyl- propan-1-ol.
References:
KARO BIO AB;ELSY, David WO2005/42464, 2005, A1 Location in patent:Page/Page column 38-39