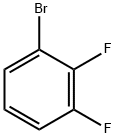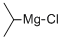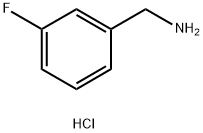
3-Fluorobenzylamine hydrochoride synthesis
- Product Name:3-Fluorobenzylamine hydrochoride
- CAS Number:658-25-3
- Molecular formula:C7H9ClFN
- Molecular Weight:161.6
100-82-3
258 suppliers
$10.00/1g

658-25-3
14 suppliers
inquiry
Yield:658-25-3 87.9 mg
Reaction Conditions:
with hydrogenchloride in water at 20;
Steps:
8. General Procedure for the Hydrosilylation of Nitriles
General procedure: A stock solution of complex E was prepared in THF from which a solution (0.025 mmol, 100 μL) was added to a screw-capped tube followed by addition of the nitrile (0.5 mmol), phenylsilane (1.5 mmol) and 0.7 mL THF under an argon atmosphere. Then the tube was sealed and heated with stirring at 120 °C (oil bath temperature) for the specified time. After that, the tube was cooled, and the reaction mixture was transferred to a round-bottomed flask where MeOH (1 mL) and 2M NaOH solution (10 mL) were slowly added with continuous stirring. The solution was stirred overnight at room temperature, followed by extraction with diethyl ether. The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in a rotary evaporator to give the crude amine product. From the crude reaction mixture, 50 μL solution was syringed out for GC analysis (n-dodecane was used as the internal standard). For isolation, the crude amine was treated with 1M HCl followed by the addition of diethyl ether, which led to precipitation of the amine salts. The precipitate was filtered off, washed with diethyl ether, and dried to obtain the pure amine salts which were confirmed by comparison of the authentic sample through NMR analyses.
References:
Ganguli, Kasturi;Mandal, Adarsha;Sarkar, Bidisha;Kundu, Sabuj [Tetrahedron,2020,vol. 76,# 37,art. no. 131439] Location in patent:supporting information
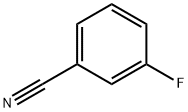
403-54-3
330 suppliers
$7.00/5g

658-25-3
14 suppliers
inquiry
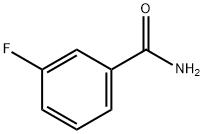
455-37-8
122 suppliers
$14.00/1g

658-25-3
14 suppliers
inquiry
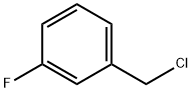
456-42-8
335 suppliers
$5.00/5g

658-25-3
14 suppliers
inquiry