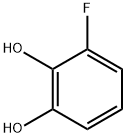
3-FLUOROCATECHOL synthesis
- Product Name:3-FLUOROCATECHOL
- CAS Number:363-52-0
- Molecular formula:C6H5FO2
- Molecular Weight:128.1
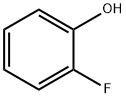
367-12-4

363-52-0
The general procedure for the synthesis of 3-fluorocatechol from 2-fluorophenol was as follows: o-fluorophenol (20 g, 0.2 mol) was dissolved in acetonitrile (500 mL) dried over molecular sieves and stirred under argon protection. Anhydrous magnesium chloride (68 g, 0.72 mol) and triethylamine (150 mL, 1.08 mol) were then added and the mixture was observed to be exothermic. After stirring for 20 minutes, paraformaldehyde (42 g) was added and the reaction mixture was kept at 50 °C for 6 hours. The reaction process was monitored by thin layer chromatography (TLC) and it was found that there were still residues of raw materials, but the prolongation of the reaction time did not significantly change the results. Upon completion of the reaction, the mixture was cooled to room temperature and an aqueous sodium hydroxide solution (22.8 g of sodium hydroxide dissolved in 80 mL of water) was slowly added under ice water bath conditions. Then 30 wt% hydrogen peroxide (140 mL) was slowly added dropwise, taking care to control the reaction temperature below 50 °C to avoid intense exotherm. After the dropwise addition, the reaction mixture was continued to be stirred at 30°C for 1.5 hours. Subsequently, the pH was adjusted to 1 with concentrated hydrochloric acid (12 mol/L) and extracted with ethyl acetate (4 x 100 mL). The organic phases were combined and saturated aqueous sodium thiosulfate (200 mL) was added and stirred for 1 hour to remove residual peroxide. After separation of the organic phase, the aqueous phase was again extracted with ethyl acetate (1 x 100 mL). All organic phases were combined, dried and concentrated with anhydrous magnesium sulfate. Finally, the residue was purified by column chromatography (eluent was petroleum ether/ethyl acetate, 15:1, v/v) to afford 10.3 g of a light yellow oily liquid (Intermediate C-1a) in 45% yield and recovered 4.3 g of o-fluorophenol.The NMR hydrogen spectral (300 MHz, CDCl3) data for the Intermediate C-1a were as follows:δ 6.63-6.45 (3H, m), δ 120.3 (d, J = 15.5 Hz), 111.6 (d, J = 2.6 Hz), 108.1 (d, J = 18.4 Hz).
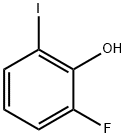
28177-50-6
70 suppliers
$62.00/1g

363-52-0
210 suppliers
$7.00/250mg
Yield:363-52-0 78%
Reaction Conditions:
Stage #1: 2-fluoro-6-iodophenolwith potassium hydroxide;water for 8 h;Heating / reflux;
Stage #2: with hydrogenchloride;water at 20; pH=2 - 3;
Steps:
5.1
1) Synthesis of 3-fluorocatechol [H]: To a 500 ml flask were added 119 g (0.5 mol) of 2-fluoro-6-iodophenol and 200 ml of a 30% aqueous potassium hydroxide solution and they were reacted under vigorous stirring under a reflux temperature for 8 hours. After the termination of the reaction, the mixture was cooled to ordinary temperature and neutralized with hydrochloric acid to adjust pH between 2 and 3. After filtration, the filtrate was washed with water to obtain 49.9 g of a product [H]. The process yield was 78%.
References:
EP2055692,2009,A1 Location in patent:Page/Page column 9

367-12-4
426 suppliers
$5.00/10g

363-52-0
210 suppliers
$7.00/250mg
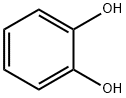
120-80-9
531 suppliers
$13.00/25g

363-52-0
210 suppliers
$7.00/250mg
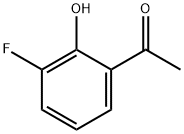
699-92-3
94 suppliers
$54.00/250mg

363-52-0
210 suppliers
$7.00/250mg
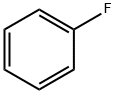
462-06-6
432 suppliers
$10.00/5g

363-52-0
210 suppliers
$7.00/250mg