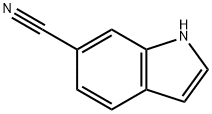
3-FORMYL-1H-INDOLE-6-CARBONITRILE synthesis
- Product Name:3-FORMYL-1H-INDOLE-6-CARBONITRILE
- CAS Number:83783-33-9
- Molecular formula:C10H6N2O
- Molecular Weight:170.17
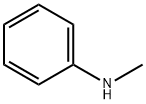
15861-36-6
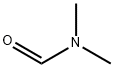
68-12-2

83783-33-9
a) Synthesis of 3-formyl-1H-indole-6-carbonitrile: Under stirring conditions, phosphorus oxychloride (POCl3, 0.593 g, 3.87 mmol) was slowly added dropwise to 5 mL of N,N-dimethylformamide (DMF). After stirring for 10 min, 6-cyanoindole (1H-indole-6-carbonitrile, 0.500 g, 3.52 mmol) was added in batches. The reaction mixture was stirred at room temperature for 1 hour, followed by warming up to 40°C and continued stirring for 1 hour. Upon completion of the reaction, the mixture was poured into ice water and adjusted to alkaline with aqueous sodium hydroxide. The mixture was then heated to 100 °C for 1 min, cooled again in an ice bath and extracted three times with ethyl acetate (EtOAc). The organic layers were combined, washed with water, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was recrystallized by water-ethanol mixed solvent to give the target product 3-formyl-1H-indole-6-carbonitrile 0.379 g in 63% yield. The product was characterized by 1H NMR (500 MHz, MeOH-d4): δ 9.98 (s, 1H), 8.33 (s, 1H), 8.31 (d, 1H), 7.89 (m, 1H), 7.52 (m, 1H).
Yield:83783-33-9 60%
Reaction Conditions:
with tert.-butylhydroperoxide;ruthenium(III)chloride hydrate;trimethylpyruvic acid in N-methyl-acetamide at 25; for 24 h;Inert atmosphere;regioselective reaction;
References:
Wu, Wenliang;Su, Weiping [Journal of the American Chemical Society,2011,vol. 133,# 31,p. 11924 - 11927] Location in patent:supporting information; experimental part

15861-36-6
219 suppliers
$5.00/1g

83783-33-9
83 suppliers
inquiry
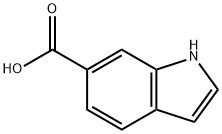
1670-82-2
351 suppliers
$10.00/1g

83783-33-9
83 suppliers
inquiry