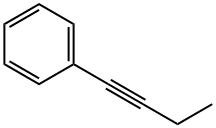
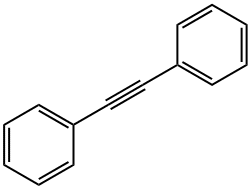
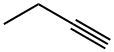
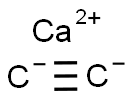3-HEXYNE synthesis
- Product Name:3-HEXYNE
- CAS Number:928-49-4
- Molecular formula:C6H10
- Molecular Weight:82.14
Yield:-
Reaction Conditions:
tris-amido molybdenum carbyne complex conjugated with silica in (2)H8-toluene at 21; for 22 h;Conversion of starting material;
Steps:
3; 8
Example 3; In situ Catalyst Preparation and Homodimerization; Amorphous silica (particle size 30-40 nm, 200 m2/g, 4 μmol/m2) was treated at 400° C. under an O2 atmosphere for 14 h. Elemental analysis of the resulting silica showed CHN values of 0.03, 0, and 0%, respectively. Inside an argon-filled glove box, silica (7.0 mg, 5.6 μmol) was added into a screw cap NMR tube. 600 μL of a yellow/brown d8-toluene solution of tris-amido Mo carbyne complex (1.67M, 1.5 μmol) was added to the NMR tube. The suspension was shaken at 25° C. for 30 min. The solution turned colorless as the silica turned brown. 1-phenyl-1-butyne (21.2 μL, 0.15 mmol) was added to the NMR tube and the suspension was shaken at 21° C. and followed up by 1HNMR. The reaction reached equilibrium after 22 min at 44% yield of diphenylacetylen and 3-hexyne with a t1/2 of less than 2.3 min.; Example 8; Catalyst Recycling; Recycling of the catalyst from Example 1 was accomplished with 0.8 mol % and 4 mol % loading. For 0.8 mol % catalyst loading, 1.0 mmol of butynyl methyl benzoate was added to a reaction vial containing 3.0 mg of the catalyst (0.50 μmol) and 600 μL of toluene and agitated for 2 h. The reaction mixture was centrifuged and decanted. The silica was washed with 200 μL of solvent, centrifuged and decanted. A second mixture of 1.0 mmol alkyne in 600 μL of toluene was added to the silica and agitated for 2 h, and repeated. Each of the cycles were analyzed and found that for cycles 1, 2, and 3 the conversions were 43.8, 32.7, and 8.7%, respectively. For the 4.0 mol % catalyst loading, 64 μmol of propynyl thiophene were added to a reaction vial containing 15.5 mg of the catalyst (2.52 μmol) and 600 μL for toluene and stirred or shaken for 5 h. The reaction mixture was centrifuged and the mixture was decanted. The silica was washed with 200 μL of solvent, centrifuged and decanted. The conversion percentages established that the catalyst may be used at least twice for the metathesis of propynyl thiophene. A second mixture of 64 μmol 1-phenyl-1-butyne in 600 μL of toluene was added to the silica and stirred or shaken for 5 h, and repeated. Each of the cycles were analyzed and found that for cycles 1, 2, and 3, the conversions were 45.2, 52.1, and 32.3% respectively. The conversion percentages established that the catalyst may be used at least three times for the metathesis of 1-phenyl-1-butyne.
References:
Weissman, Haim;Plunkett, Kyle N.;Cho, Hyeon Mo;Moore, Jeffrey S. US2006/281938, 2006, A1 Location in patent:Page/Page column 7; 8