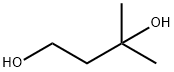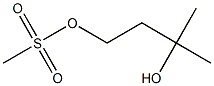
3-hydroxy-3-methylbutyl methanesulfonate synthesis
- Product Name:3-hydroxy-3-methylbutyl methanesulfonate
- CAS Number:135859-47-1
- Molecular formula:C6H14O4S
- Molecular Weight:182.238
Yield:135859-47-1 72%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 7.5 h;Cooling with ice;
Steps:
4.1.1.72. 3-Hydroxy-3-methylbutyl methanesulfonate
3-Methyl-1,3-butandiol (3.83 g, 36.8 mmol) was dissolved in CH2Cl2 (150 ml). Triethylamine (6.66 ml, 47.8 mmol) and methanesulfonyl chloride (3.13 ml, 40.5 mmol) were added to the ice-cooled solution, and stirred for 7.5 h at room temperature. Water (100 ml) was added, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2. Then, combined organic layer was washed with brine, and dried over Na2SO4. The solvent was removed in a reduced presser to give title compound (4.79 g, 26.3 mmol, 72%) as colorless oil. 1H NMR (CDCl3) δ 1.30 (6H, s), 1.96 (2H, t, J = 6.8 Hz), 3.02 (3H, s), 4.42 (2H, t, J = 6.8 Hz)
References:
Ichikawa, Masanori;Yokomizo, Aki;Itoh, Masao;Haginoya, Noriyasu;Sugita, Kazuyuki;Usui, Hiroyuki;Terayama, Koji;Kanda, Akira [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 17,p. 5207 - 5224] Location in patent:experimental part