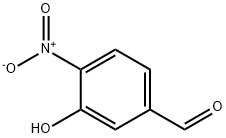
3-Hydroxy-4-nitrobenzaldehyde synthesis
- Product Name:3-Hydroxy-4-nitrobenzaldehyde
- CAS Number:704-13-2
- Molecular formula:C7H5NO4
- Molecular Weight:167.12

100-83-4
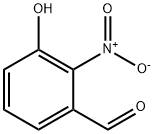
42123-33-1

704-13-2
General procedure for the synthesis of 3-hydroxy-2-nitrobenzaldehyde and 3-hydroxy-4-nitrobenzaldehyde from m-hydroxybenzaldehyde: To a stirred solution of m-hydroxybenzaldehyde (618 mg, 5.0 mmol) in methylene chloride (10 mL) was added tetrabutylammonium hydrogensulfate (85.0 mg, 0.25 mmol) and isopropyl nitrate (1.27 mL, 12.5 mmol). Concentrated sulfuric acid (610 μL) was added slowly dropwise and the reaction mixture was stirred for 15 min at room temperature. Upon completion of the reaction, the mixture was transferred to a split funnel containing 50 mL of saturated aqueous sodium bicarbonate solution and the crude product was extracted with dichloromethane. The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated in vacuum. The resulting solid was adsorbed on silica gel and purified by fast column chromatography with an eluent ratio of 99:1 to 4:1 hexane:ethyl acetate, which first gave 3-hydroxy-4-nitrobenzaldehyde (Rf = 0.44, eluent ratio 3:1 hexane:ethyl acetate) as a yellow solid (201 mg, 24% yield); followed by 3-hydroxy-2-nitrobenzaldehyde (Rf = 0.19, eluent ratio 3:1 hexane:ethyl acetate) as a light yellow solid (411 mg, 47% yield). This method is referenced from D.A. Learmonth et al. (GB-2377934, 2003). In this study, the yield of 3-hydroxy-2-nitrobenzaldehyde was increased to 63% (CAS No. 42123-33-1). Compound 28 (3-hydroxy-2-nitrobenzaldehyde) was characterized by the following data: 1H NMR (400 MHz, CDCl3): δ 10.40 (s, 1H), 10.30 (s, 1H), 7.67 (dd, J = 8.3, 7.4, 0.7 Hz, 1H), 7.37 (dd, J = 8.3, 1.4 Hz, 1H), 7.31 ( dd, J = 7.4, 1.4 Hz, 1H). HRMS (DART): calculated value C7H9N2O4 [M + NH4]+: 185.0557, measured value: 185.0559. melting point: 155-158°C, literature value: 157°C (W.S. Saari et al., J. Med. Chem. 1974, 17, 1086 -1090). Compound 30 (3-hydroxy-4-nitrobenzaldehyde) was characterized by the following data: 1H NMR (400 MHz, CDCl3): δ 10.58 (s, 1H), 10.06 (d, J = 0.6 Hz, 1H), 8.28 (d, J = 8.7 Hz, 1H), 7.66 (d, J = 1.7 Hz, 1H), 7.51 (dd, J = 8.7, 1.7 Hz, 1H), in agreement with 1H NMR data reported in the literature (A. Tsoukala et al., Tet. Lett. 2009, 50, 831-833). Melting point: 129-131°C, literature value: 127°C (J. Cologne et al., Bull. Soc. Chim. Fr. 1964, 12, 3090-3096).
619-14-7
303 suppliers
$10.00/5g

704-13-2
149 suppliers
$30.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 87 percent / 10 M BH3*SMe2 / tetrahydrofuran / 3 h / Heating
2: 100 percent / MnO2 / CH2Cl2 / 20 h
References:
Olszewski;Marshalla;Sabat;Sundberg [Journal of Organic Chemistry,1994,vol. 59,# 15,p. 4285 - 4296]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

704-13-2
149 suppliers
$30.00/1g

100-83-4
647 suppliers
$5.00/10g

704-13-2
149 suppliers
$30.00/1g
61161-83-9
42 suppliers
$20.00/1g

704-13-2
149 suppliers
$30.00/1g