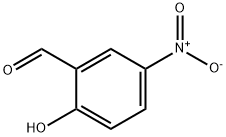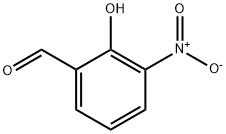
3-Nitrosalicylaldehyde synthesis
- Product Name:3-Nitrosalicylaldehyde
- CAS Number:5274-70-4
- Molecular formula:C7H5NO4
- Molecular Weight:167.12
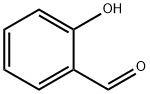
90-02-8

5274-70-4
GENERAL METHOD: Salicylaldehyde (10 mmol) was mixed with urea nitrate (10 mmol) in acetonitrile-water solvent mixture (95:5, 5 ml, v/v) in a 25 ml round bottom flask. The reaction mixture was placed in a Milestone Start SYNTH microwave reactor and heated at 80°C for 40-50 min. Upon completion of the reaction, the reaction mixture was cooled to room temperature, quenched with water and extracted with dichloromethane. The organic phase was concentrated under reduced pressure and the residue was purified by silica gel column chromatography to afford the target product 3-nitrosalicylaldehyde. In all experiments, o-nitrosalicylaldehyde was selectively generated and no para-nitro-substituted by-products were detected. The resulting compounds were characterized by 1H NMR, 13C NMR, melting point determination (for solid samples), GC-MS analysis and comparison with standards.

90-02-8
478 suppliers
$9.00/1g

5274-70-4
205 suppliers
$67.00/1g
Yield:5274-70-4 62%
Reaction Conditions:
with uronium nitrate in water;acetonitrile at 80; for 0.75 h;Microwave irradiation;regioselective reaction;
Steps:
General procedure: Phenol (10 mmol) and urea nitrate (10 mmol)were mixed together in acetonitrile-water (95:5, 5 ml) in a 25 ml round bottomed flask and placed in a Milestone’s Start SYNTH microwave reactor. The reaction mixture was heated at 80°C for 40-50 min. At the end of the reaction, the reaction mixture was allowed to cool at room temperature,treated with water, and extracted with dichloromethane. After removing the solvent under reduced pressure, the residue was purified by column chromatography on silica gel to give the corresponding nitrophenol. In allcases ortho-nitrophenols were obtained selectively without any evidence forthe formation of the para-substituted nitrophenols. All the compoundsobtained were characterized by 1H NMR, 13C NMR, mp (for solids), GC-MSand in comparison with authentic samples.
References:
Verma, Sanny;Pandita, Sangeeta;Jain, Suman L. [Tetrahedron Letters,2014,vol. 55,# 7,p. 1320 - 1322]
![2-[(hydroxyimino)methyl]-6-nitrophenol](/CAS/20210111/GIF/17580-64-2.gif)
17580-64-2
6 suppliers
inquiry

5274-70-4
205 suppliers
$67.00/1g
89-95-2
211 suppliers
$22.00/10g

5274-70-4
205 suppliers
$67.00/1g