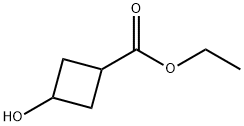
3-Hydroxy-cyclobutanecarboxylic acid ethyl ester synthesis
- Product Name:3-Hydroxy-cyclobutanecarboxylic acid ethyl ester
- CAS Number:17205-02-6
- Molecular formula:C7H12O3
- Molecular Weight:144.17
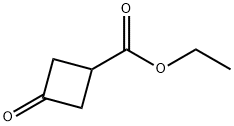
87121-89-9

17205-02-6
General procedure for the synthesis of ethyl 3-hydroxycyclobutanecarboxylate from ethyl 3-oxocyclobutanecarboxylate: to a solution of ethyl 3-oxocyclobutanecarboxylate (0.18 g, 1.27 mmol) in methanol (10 mL) was slowly added sodium borohydride (0.048 g, 1.27 mmol) at 0 °C. The reaction mixture was stirred continuously at 0 °C for 30 min, followed by quenching the reaction with aqueous 1N hydrochloric acid. Volatile solvents were removed by distillation under reduced pressure and the residue was extracted by partitioning with ethyl acetate and 1N aqueous hydrochloric acid. The organic phase was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to afford the target product ethyl 3-hydroxycyclobutanecarboxylate (0.17 g, 93% yield) as a mixture of cis and trans isomers. The product was characterized by 1H NMR (500 MHz, CDCl3), δ 4.17-4.24 (m, 1H), 4.15 (d, J=7.2 Hz, 2H), 2.54-2.66 (m, 3H), 2.11-2.22 (m, 2H), 1.27 (t, J=7.2 Hz, 3H).

87121-89-9
150 suppliers
$9.00/1g

17205-02-6
101 suppliers
$6.00/100mg
Yield: 93%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0; for 0.5 h;
Steps:
53.53B 53B. Ethyl 3 -hydroxy cyclobutanecarboxylate
53B. Ethyl 3 -hydroxy cyclobutanecarboxylate [00218] To a 0 °C solution of ethyl 3-oxocyclobutanecarboxylate (0.18 g, 1.27 mmol) in MeOH (10 mL) was added NaBH4 (0.048 g, 1.27 mmol). The solution was stirred at 0 °C for 30 min and then quenched with 1 N aq. HC1. Volatiles were removed in vacuo, and the residue was partitioned between EtOAc and 1 N aq. HC1. The organic layer was dried (MgS04) and concentrated in vacuo to afford the title compound (0.17 g, 93% yield) as a mixture of the cis- and trans-isomers. lR NMR (500 MHz, CDCI3) δ 4.17 - 4.24 (m, 1H), 4.15 (d, J = 7.2 Hz, 2H), 2.54 - 2.66 (m, 3H), 2.1 1 - 2.22 (m, 2H), 1.27 (t, J = 7.2 Hz, 3H).
References:
BRISTOL-MYERS SQUIBB COMPANY;SHI, Yan;WANG, Ying;CHENG, Peter T.W.;WU, Shung C. WO2016/40223, 2016, A1 Location in patent:Paragraph 00218
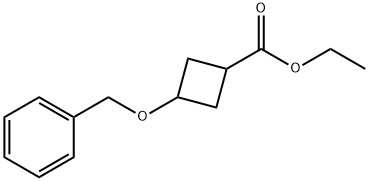
106596-81-0
23 suppliers
$293.32/0.5g

17205-02-6
101 suppliers
$6.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17205-02-6
101 suppliers
$6.00/100mg
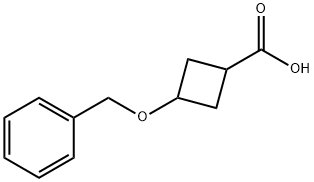
4958-02-5
97 suppliers
$29.00/1g

17205-02-6
101 suppliers
$6.00/100mg
23761-23-1
486 suppliers
$6.00/1g

17205-02-6
101 suppliers
$6.00/100mg