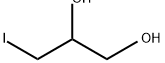
3-iodopropane-1,2-diol synthesis
- Product Name:3-iodopropane-1,2-diol
- CAS Number:554-10-9
- Molecular formula:C3H7IO2
- Molecular Weight:201.99
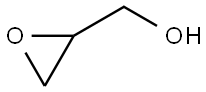
556-52-5
296 suppliers
$17.67/1gm:

554-10-9
23 suppliers
$555.45/500MG
Yield:554-10-9 100 %
Reaction Conditions:
with acetic acid;lithium iodide in tetrahydrofuran at 20;Inert atmosphere;
Steps:
3-Iodopropane-1,2-diol (9)
LiI (80.0 mmol, 10 g) was added to a solution of glycidol(50.0 mmol) and acetic acid (150 mmol) in anhydrous THF (40 mL), and the solution waskept at room temperature and stirred in argon atmosphere for 40 min. The mixture wasdiluted with distilled water and extracted with two aliquots of ethyl acetate (2 20 mL).The organic layer was treated with anhydrous sodium sulphate and filtered, and thesolvent was removed using a rotary evaporator and high-vacuum pump. By followingthis procedure, 3-iodopropane-1,2-diol (9) was obtained as a yellow amorphous solid(50.0 mmol, quant.) 1H NMR (400 MHz, D2O) 3.59-3.42 (m, 3H), 3.25 (dd, J = 10.8, 4.5 Hz,1H), 3.15 (dd, J = 10.8, 4.5 Hz, 1H). 13C{1H} NMR (101 MHz, D2O) 70.6, 64.4, 8.6. HRMS(ESI) m/z: [M + H]+ calcd for C3H8IO2+ 202.9563 found 202.9554
References:
Poletti, Lorenzo;Rovegno, Caterina;Di Carmine, Graziano;Vacchi, Filippo;Ragno, Daniele;Brandolese, Arianna;Massi, Alessandro;Dambruoso, Paolo [Molecules,2023,vol. 28,# 4,art. no. 1530]
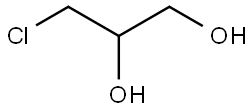
96-24-2
303 suppliers
$11.00/5g

554-10-9
23 suppliers
$555.45/500MG
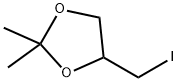
23737-52-2
4 suppliers
inquiry

554-10-9
23 suppliers
$555.45/500MG