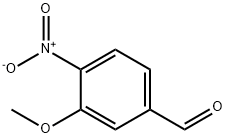
3-METHOXY-4-NITROBENZALDEHYDE synthesis
- Product Name:3-METHOXY-4-NITROBENZALDEHYDE
- CAS Number:80410-57-7
- Molecular formula:C8H7NO4
- Molecular Weight:181.15
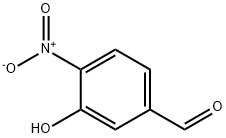
704-13-2

74-88-4

80410-57-7
(1) Synthesis of 3-methoxy-4-nitrobenzaldehyde: 3-hydroxy-4-nitrobenzaldehyde (500 mg, 2.99 mmol) and potassium carbonate (0.42 g, 3.0 mmol) were dissolved in 6.0 mL of N,N-dimethylformamide. Iodomethane (0.38 mL, 6.0 mmol) was slowly added to the reaction system at room temperature, followed by continuous stirring at the same temperature for 5 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and water and separated. The organic layer was washed twice with saturated brine and subsequently dried with anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 4/1 to 1/1) to afford the target compound, 3-methoxy-4-nitrobenzaldehyde, as a light yellow powder (518 mg, 96% yield).
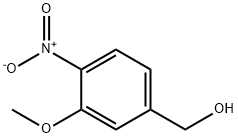
80866-88-2
66 suppliers
$43.00/250mg

80410-57-7
48 suppliers
inquiry
Yield: 100%
Reaction Conditions:
with dipyridinium dichromate in dichloromethane at 20; for 17 h;
Steps:
C 3-Methoxy-4-nitrobenzaldehyde (44):
A dispersion of (3-methoxy-4-nitrophenyl)methanol (43) (1.4 mmol) and pyridinium dichromate (2.3 mmol) in dry DCM(38 mL) was stirred 17 hours at room temperature and a mixture of celite and silica was added.
The dispersion was filtered and washed with DCM (3x10 mL).
The solvent of the resulting solution was evaporated under vacuum to give a brown residue (1.3 mmol, quantitative yield).
1H NMR (400 MHz, CDCl3) δ 10.05 (s, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.60 (d, J = 1.3 Hz, 1H), 7.54 (dd, J = 8.1, 1.5 Hz, 1H), 4.03 (s, 3H).
References:
Consejo Superior De Investigaciones Científicas;Universitat Autònoma De Barcelona;Institució Catalana de Recerca i Estudis Avançats;Fundació Institut de Bioenginyeria de Catalunya;Centre National de la Recherche Scientifique (CNRS);Llebaria Soldevila, Amadeo;Gorostiza Langa, Pau;Gomez Santacana, Xavier;Pittolo, Silvia;Giraldo Arjonilla, Jesús;Rovira Algans, Xavier;Goudet, Cyril;Pin, Jean Philippe EP2853565, 2015, A1 Location in patent:Paragraph 0220
591-31-1
432 suppliers
$10.00/5g

80410-57-7
48 suppliers
inquiry

591-31-1
432 suppliers
$10.00/5g

80410-57-7
48 suppliers
inquiry

100-83-4
648 suppliers
$5.00/10g

80410-57-7
48 suppliers
inquiry