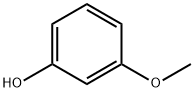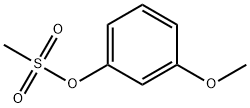
3-Methoxyphenyl methanesulfonate synthesis
- Product Name:3-Methoxyphenyl methanesulfonate
- CAS Number:52200-03-0
- Molecular formula:C8H10O4S
- Molecular Weight:202.23
Yield:52200-03-0 90%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
General procedure for synthesis of aryl mesylates
General procedure: To a solution of phenol substrate (1.0 eq, 10.0 mmol) and Et3N (1.5 eq, 15.0 mmol) in CH2Cl2 (0.5 g/mL) was added dropwise the solution of MsCl (1.2 eq, 12.0 mmol) in CH2Cl2 at 0 oC. The mixture was allowed to warm to RT and stirred for 4 h-12 h to detect for the reaction completion. The reaction solution was added to H2O (20 ml). The layers were separated and the aqueous layer was extracted with CH2Cl2 (10 mL× 2). The combined organic layers were washed with brine (20 mL), and dried over Na2SO4. After the solvent was evaporated, the product was purified by column chromatography over silica gel.
References:
Tu, Yahui;Zhang, Yi;Xu, Sheng;Zhang, Zhaoguo;Xie, Xiaomin [Synlett,2014,vol. 25,# 20,art. no. ST-2014-W0578-L,p. 2938 - 2942] Location in patent:supporting information