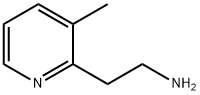
3-METHYL-2-PYRIDINEETHANAMINE synthesis
- Product Name:3-METHYL-2-PYRIDINEETHANAMINE
- CAS Number:851670-19-4
- Molecular formula:C8H12N2
- Molecular Weight:136.19
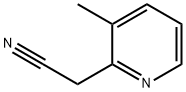
38203-11-1
21 suppliers
inquiry

851670-19-4
26 suppliers
$45.00/10mg
Yield:851670-19-4 86%
Reaction Conditions:
Stage #1: (3-methyl-pyridin-2-yl)-acetonitrilewith borane-THF in tetrahydrofuran;water at 20 - 130; for 0.116667 h;Microwave;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20 - 120; for 0.0666667 h;Microwave;
Steps:
129.a
2-(3-Methylpyridin-2-yl)ethyl amine: To a solution of 2-(3-methylpyridine-2-yl)acetonitrile (example 129b) (95 mg, 0.72 mmol) in THF (0.5 mL) was added 1 M BH3.THF (2.2 mL, 2.2 mmol) dropwise at room temperature. The resulting mixture was heated in a microwave reactor at 130° C. for 7 min. Then, 6 N aqueous HCl (1 mL) was added dropwise at room temperature. The resulting mixture was heated in a microwave reactor at 120° C. for 4 min. The reaction mixture was washed with Et2O (3×3 mL), then cooled to 0° C. and 10 N aqueous NaOH (0.8 mL) was added. The aqueous solution was saturated with K2CO3. The product was extracted with CHCl3 (6×5 mL). The organic extracts were dried (1:1 K2CO3/Na2SO4), filtered, concentrated in vacuo to afford an oil (85 mg, 86%), which was used directly in Example 8. m/e=137 [M+1].
References:
US2005/84506,2005,A1 Location in patent:Page/Page column 79