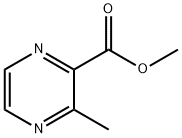
3-METHYLPYRAZINE-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:3-METHYLPYRAZINE-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:41110-29-6
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15

67-56-1
41110-28-5

41110-29-6
3-Methylpyrazine-2-carboxylic acid (19.95 g, 144 mmol) was suspended in methanol (500 mL) in a 2L round bottom flask. The suspension was cooled in an ice-water bath, followed by slow dropwise addition of concentrated sulfuric acid (27.3 mL, 506 mmol) over 5 min. The reaction mixture was heated to 80 °C and maintained at this temperature for 5 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue was dissolved in dichloromethane (750 mL). Excess acid was neutralized in small amounts with 5 M aqueous sodium hydroxide solution (200 mL). The aqueous layer was separated and extracted with dichloromethane (250 mL). All organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated to give methyl 3-methylpyrazine-2-carboxylate (16.15 g, 106 mmol, 73% yield). Mass spectral analysis showed m/z = 153 [M + H]+.

67-56-1
790 suppliers
$7.29/5ml-f

41110-28-5
135 suppliers
$8.00/250mg

41110-29-6
96 suppliers
$9.00/250mg
Yield: 87%
Reaction Conditions:
with sulfuric acid at 0 - 60; for 4 h;Inert atmosphere;
Steps:
A Step A:
Methyl 3-methylpyrazine-2-carboxylate.
To a solution of 3-methylpyrazine-2-carboxylic acid (6 g, 43.4 mmol, 1 equiv) in methanol (60 mL) at 0° C. was added H2SO4 (2.9 mL, 54.3 mmol, 1.25 equiv) dropwise, and the reaction mixture was stirred at 60° C. for 4 h.
The solvent was evaporated, and the residue was dissolved in water (5 ml) and basified with a 10% aq. Na2CO3 solution to pH 12.
The solution was extracted with AcOEt.
The combined organic layers were dried over MgSO4, filtered and the solvents evaporated under vacuum to give the title compound (5.75 g, 37.8 mmol, 87%), which was used in the next step without further purification. 1H NMR (300 MHz, CDCl3) δ 8.61 (s, 1H), 8.51 (s, 1H), 4.01 (s, 3H), 2.85 (s, 3H).
References:
JANSSEN PHARMACEUTICA NV;GELIN, Christine US2020/392155, 2020, A1 Location in patent:Paragraph 0172; 0209-0210

41110-28-5
135 suppliers
$8.00/250mg

41110-29-6
96 suppliers
$9.00/250mg