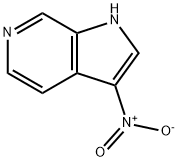
3-NITRO-6-AZAINDOLE synthesis
- Product Name:3-NITRO-6-AZAINDOLE
- CAS Number:67058-77-9
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
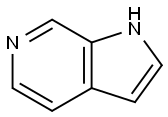
271-29-4

67058-77-9
General procedure for the synthesis of 3-nitro-1H-pyrrolo[2,3-c]pyridine from 6-azaindole: 69% nitric acid (533 mg, 8.47 mmol) was slowly added to a solution of 1H-pyrrolo[2,3-c]pyridine (1 g, 8.47 mmol) in concentrated sulfuric acid (5 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 2 h before gradually warming to room temperature and continuing to stir overnight. Upon completion of the reaction, the mixture was carefully poured into water (100 mL) and the pH was adjusted with sodium hydroxide powder to >7. Subsequently, the mixture was extracted with ethyl acetate (100 mL x 3), the organic phases were combined and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to afford 3-nitro-1H-pyrrolo[2,3-c]pyridine (690 mg, 50% yield) as a yellow solid. The product was confirmed by NMR hydrogen spectroscopy (400 MHz, DMSO-d6): δ=8.94 (s, 1H), 8.85 (s, 1H), 8.44 (d, J=5.2 Hz, 1H), 8.02 (dd, J=5.6,1.2 Hz, 1H). Mass spectral analysis showed m/z of 164.0 (M+H).

271-29-4
272 suppliers
$9.00/250mg

67058-77-9
30 suppliers
inquiry
Yield:67058-77-9 50%
Reaction Conditions:
with sulfuric acid;nitric acid at 0 - 20;
Steps:
349.1 Step 1
To a solution of 1H-pyrrolo[2,3-c]pyridine (1 g, 8.47 mmol) in H2S04 (5 mL) was added 69% HNO3 (533 mg, 8.47 mmol) at 0 °C. After stirred at 0 °C for 2 hrs, the reaction mixture was allowed to warm to room temperature and stirred overnight. The mixture was then poured into H20 (100 mL) and basified by NaOH powder to pH> 7. Then the mixture was extracted by EA (100 mL x3), dried over anhydrous Na2SO4, and evaporated in vacuum to afford 3-nitro-1H- pyrrolo[2,3-c]pyridine (690 mg, 50%) as a yellow solid. ‘H NIVIR (400 IVIHz, DMSO-d6): ? = 8.94 (s, 1H), 8.85 (s, 1H), 8.44 (d, J= 5.2 Hz, 1H), 8.02 (dd, J= 5.6, 1.2 Hz, 1H). MS: m/z 164.0 (M+H).
References:
WO2018/132372,2018,A1 Location in patent:Paragraph 00729