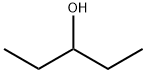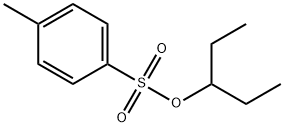
3-Pentanol, 4-methylbenzenesulfonate synthesis
- Product Name:3-Pentanol, 4-methylbenzenesulfonate
- CAS Number:950-25-4
- Molecular formula:C12H18O3S
- Molecular Weight:242.33
Yield:950-25-4 86%
Reaction Conditions:
with pyridine at 0 - 20;
Steps:
57 Example 57: 5-((5-methyl-4-(pentan-3-ylthio)pyrimidin-2- yl)amino)benzo[c][1,2]oxaborol-1(3H)-
To a solution of pentan-3-ol (5.7 g, 60mmol) in anhydrous pyridine (40 mL) was added slowly p-toluenesulfonyl chloride (13.6 g; 66 mmol) at 0°C. The solution was warmed to room temperature and the mixture was stirred overnight. Hexane (30 mL) was added and the solution was filtered. The solid was washed with hexane (30 mL). The combined organic layers were washed with aqueous hydrochloric acid (5N, 2 × 30 mL). After drying over anhydrous sodium sulfate and filtration, the solvent was removed by rotary evaporation to give pentan-3-yl 4- methylbenzene sulfonate as a white solid (11.7 g, yield 86%). Potassium thioacetate (5.71 g, 50 mmol) was dissolved in dimethylformamide (20 mL). Pentan-3-yl 4-methylbenzene sulfonate (11.4g, 50 mmol) was added and the solution was stirred at 80°C for 2 hours. Then saturated aqueous sodium chloride solution (100 mL) was added. The aqueous solution was extracted with diethyl ether (3 × 100 mL) and the combined organic layers were washed with saturated aqueous sodium chloride solution (5 × 50 mL). After drying over anhydrous sodium sulfate and filtration, the solvent was removed by rotary evaporation to give S-(pentan-3-yl) ethanethioate as a red oil (5.6 g, yield 72%).1H NMR (400 MHz, CDCl3): d 3.38-3.31 (m, 1H), 2.25 (s, 3H), 1.64-1.45 (m, 4H), 0.87 (t, J = 7.4 Hz, 6H) ppm. This ester intermediate (5.6 g, 38 mmol) was dissolved in anhydrous diethyl ether (15 mL), and was reduced by slowly adding a suspension of lithium aluminum hydride (2.81 g, 76 mmol) in anhydrous diethyl ether (20 mL) at 0°C. The solution was stirred for 2 hours at room temperature. Saturated aqueous ammonium chloride solution (20 mL) was added slowly at 0°C, and then Na2SO4 was added. The mixture was stirred for 10 minutes and then filtered through a Celite pad. The filter cake was washed with diethyl ether (3×20 mL), and the filtrate was concentrated in vacuo under low temperature to remove the solvent and to give the residue, pentane-3-thiol, as a colorless oil (2.78g, yield 71%) which was used in the next step without further purification. To a solution of 2,4-dichloro-5- methylpyrimidine (4.0 g, 25.0 mmol) and pentane-3-thiol (2.78 g, 25 mmol) in (0887) dimethylformamide (20 mL) was added NaH (2.0 g, 50.0 mmol) in portions at room temperature. The reaction was stirred at 60oC overnight, and then the reaction was quenched by water. (0888) Normal work-up as described in Example 41 gave a residue, which was purified by column chromatography to give 2-chloro-5-methyl-4-(pentan-3-ylthio)pyrimidine (2.45 g, yield 44%) as a white solid.1H NMR (400 MHz, CDCl3): d 7.93 (s, 1H), 3.97-3.89 (m, 1H), 2.08 (s, 3H), 1.78- 1.60 (m, 4H), 0.94 (t, J = 7.4 Hz, 6H) ppm. To a solution of this thioether intermediate (1.0 g, 4.6 mmol) in ethanol (8 mL) were added methyl 5-amino-2-bromobenzoate (1.05 g, 4.6 mmol) and HCl (1.5 N, 4 mL). The reaction was refluxed for 3 hours. Normal work-up as described in Example 41 generated the residue, which was triturated with ethyl acetate:petroleum ether (1:5, v/v) to give methyl 2-bromo-5-((5-methyl-4-(pentan-3-ylthio)pyrimidin-2-yl)amino)benzoate as a white solid.1H NMR (400 MHz, CDCl3): d 8.08 (d, J = 2.7 Hz, 1H), 8.06 (br. s, 1H), 7.85 (s, 1H), 7.65 (dd, J = 8.7, 2.7 Hz, 1H), 7.59 (d, J = 8.7 Hz, 1H), 4.10-3.96, (m, 1H), 3.95 (s, 3H), 2.14 (s, 3H), 1.88- 1.66 (m, 4H), 1.02 (t, J = 7.4 Hz, 6H) ppm. To a solution of this bromo intermediate (1.2 g, 2.8 mmol) in 1,4-dioxane (10 mL) were added potassium acetate (824 mg, 8.4 mol), (BPin)2 (1.06 mg, 4.2 mmol) and Pd(dppf)Cl2 (457 mg, 0.6 mmol) at room temperature under nitrogen atmosphere. The mixture was stirred at 100oC overnight, and then the solid was filtered. The residue after evaporation of the filtrate was purified by silica gel chromatography by elution with petroleum ether/ethyl acetate (100/1 to 20/1, v/v) to give methyl 5-((5-methyl-4- (pentan-3-ylthio)pyrimidin-2-yl)amino)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (1.1 g) as a white solid.1H NMR (400 MHz, CDCl3) d 8.09 (d, J = 2.1 Hz, 1H), 7.81 (s, 1H), 7.70 (dd, J = 8.1, 2.2 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.22 (s, 1H), 4.05-3.96, (m, 1H), 3.84 (s, 3H), 2.03 (s, 3H), 1.77-1.60 (m, 4H), 1.34 (s, 12H), 0.93 (t, J = 7.4 Hz, 6H) ppm. To a solution of this boron intermediate (1.1 g, 2.3 mmol) in metanol (15 mL) was added NaBH4 (532 mg, 14.0 mmol) at 30oC in small portions. The reaction was stirred at 30oC for 30 minutes, and 6N HCl (3 mL) was added. It was stirred for another 20 minutes, and then neutralized with saturated NaHCO3. Normal work-up as described in Example 41 generated the residue, which was recrystallized with dimethyl sulfoxide and water to give the product 5-((5-methyl-4-(pentan-3- ylthio)pyrimidin-2-yl)amino)benzo[c][1,2] oxaborol-1(3H)-ol (208 mg, yield 25%) as a white solid. 1H NMR (400 MHz, DMSO-d6): d 9.65 (s, 1H), 8.88 (br. s, 1H), 8.05 (s, 1H), 7.87 (s, 1H), 7.61 (d, J = 0.7 Hz, 2H), 4.95 (s, 2H), 4.12-3.98 (m, 1H), 2.05 (s, 3H), 1.86-1.62 (m, 4H), 0.97 (t, J = 7.3 Hz, 6H) ppm. HPLC purity: 96.56% at 210 nm and 96.81% at 254 nm. MS: m/z = 344.1 (M+H)+.
References:
WO2021/3501,2021,A1 Location in patent:Paragraph 0633-0635