
3-PENTYLOXY-BENZALDEHYDE synthesis
- Product Name:3-PENTYLOXY-BENZALDEHYDE
- CAS Number:24083-06-5
- Molecular formula:C12H16O2
- Molecular Weight:192.25
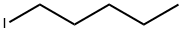
628-17-1
156 suppliers
$29.00/5g

100-83-4
649 suppliers
$5.00/10g

24083-06-5
23 suppliers
$150.00/500mg
Yield:24083-06-5 75%
Reaction Conditions:
Stage #1: meta-hydroxybenzaldehydewith potassium carbonate in acetone at 0; for 0.25 h;Inert atmosphere;
Stage #2: amyl iodide in acetone; for 3.5 h;Inert atmosphere;Reflux;
Steps:
To a solution of K2CO3 (3.40 g, 24.6 mmol) in acetone (10 mL), 3-hydroxybenzaldehyde (21) (2.00 g, 16.4 mmol) was added at 0°C under an argon atmosphere and then the mixture was stirred for 15 min. After 1-iodopentane (6.50 g, 32.8 mmol) was added to the solution, the resulting mixture was refluxed for 3.5 h. The reaction was filtered and the filtrate was evaporated to eliminate acetone. The resulting residue was diluted with H2O and extracted with EtOAc. The organic layers were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (EtOAc-hexane, 30 : 70) to afford 3-(pentyloxy)benzaldehyde (22) (2.36 g, 12.3 mmol, 75%) as a colorless oil: 1H-NMR (CDCl3, 270 MHz) δ: 0.94 (3H, t, J=7.0 Hz, -CH3), 1.33-1.52 (4H, m, -CH2-), 1.75-1.87 (2H, m, -CH2-), 4.02 (2H, t, J=6.6 Hz, -OCH2-), 7.17 (1H, m, Ar-H), 7.38 (1H, m, Ar-H), 7.41-7.48 (2H, m, Ar-H), 9.97 (1H, s, -CHO). The spectral data were in agreement with those in the literature.21)
References:
Okuda, Katsuhiro;Nishikawa, Keisuke;Fukuda, Hiroshi;Fujii, Yoshiharu;Shindo, Mitsuru [Chemical and Pharmaceutical Bulletin,2014,vol. 62,# 6,p. 600 - 607]
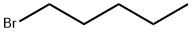
110-53-2
412 suppliers
$13.69/5g

100-83-4
649 suppliers
$5.00/10g

24083-06-5
23 suppliers
$150.00/500mg

71-41-0
449 suppliers
$10.00/25mL

100-83-4
649 suppliers
$5.00/10g

24083-06-5
23 suppliers
$150.00/500mg