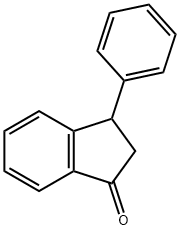
3-PHENYL-1-INDANONE synthesis
- Product Name:3-PHENYL-1-INDANONE
- CAS Number:16618-72-7
- Molecular formula:C15H12O
- Molecular Weight:208.26

30516-40-6
5 suppliers
inquiry

16618-72-7
77 suppliers
$16.00/100mg
Yield: 82%
Reaction Conditions:
with potassium phosphate;copper(l) iodide;1,10-Phenanthroline in 1,4-dioxane at 80;Schlenk technique;Solvent;Time;
Steps:
General Procedure-2 [For the open air oxidation of alcohols to carbonyl compounds; GP-2(method B)]:
General procedure: In an oven dried Schlenk tube, were added alcohol 1 (69.0-199.5 mg, 0.5 mmol), CuI (10 mol%)and 1,10-Phenanthroline (20 mol%) and K3PO4 (2 mmol) followed by the addition of dioxane (2mL) at room temperature under open air atmosphere. The stirred reaction mixture was heated inan oil bath at 80 C for 7-48 h. Progress of the reaction was monitored by TLC till the reaction iscompleted. Then, the reaction mixture was cooled to room temperature, quenched with aqueousNH4Cl solution and then extracted with CH2Cl2 (3 10 mL). The organic layer was washed withsaturated NaCl solution, dried (Na2SO4), and filtered. Evaporation of the solvent under reducedpressure and purification of the crude material by silica gel column chromatography (petroleumether/ethyl acetate) furnished the aldehyde/ketone 2 (61-97%).
References:
Reddy, Alavala Gopi Krishna;Mahendar, Lodi;Satyanarayana, Gedu [Synthetic Communications,2014,vol. 44,# 14,p. 2076 - 2087] Location in patent:supporting information
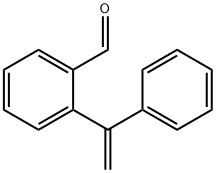
871563-46-1
0 suppliers
inquiry

16618-72-7
77 suppliers
$16.00/100mg
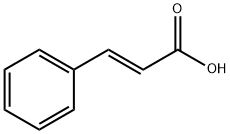
140-10-3
643 suppliers
$5.00/10g

16618-72-7
77 suppliers
$16.00/100mg
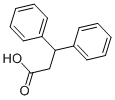
606-83-7
215 suppliers
$10.00/5g

16618-72-7
77 suppliers
$16.00/100mg

140-10-3
643 suppliers
$5.00/10g
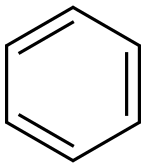
71-43-2
721 suppliers
$11.19/25ML

16618-72-7
77 suppliers
$16.00/100mg