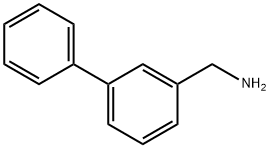
3-PHENYLBENZYLAMINE synthesis
- Product Name:3-PHENYLBENZYLAMINE
- CAS Number:177976-49-7
- Molecular formula:C13H13N
- Molecular Weight:183.25
4152-90-3
218 suppliers
$6.00/1g

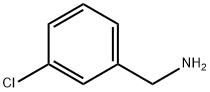
98-80-6
743 suppliers
$5.00/5g

177976-49-7
76 suppliers
$55.00/250mg
Yield:177976-49-7 95 %Chromat.
Reaction Conditions:
with palladium diacetate;2-(di-tert-butyl-phosphino)-1-phenyl-1H-pyrrole in water at 100; for 4 h;Inert atmosphere;Suzuki Coupling;
Steps:
4
General procedure: [0041] Equation 7A mixture of aryl chlorides (10 mmol, 1.0 equiv, listed in Table 5), phenylboronic acid (1.829 g, 15 mmol, 1.5 equiv), Pd(OAc)2 (as listed in Table 5), PtB (as listed in Table 5) and 0 (25 mL, 1384 mmol) in a 3-neck round-bottom flask was stirred under reflux at 100 °C in a N2 atmosphere for the time listed in Table 5. The initial and final pH values of the aqueous phase was measured before and after the heating was enabled when the temperature was stabilized at 25 - 27 °C, as reported in Table 5. The initial reaction time (t = 0) was taken when the reaction mixture reached an internal temperature of 100 °C; the time to reach this temperature was approximately 15-20 minutes. The cooled reaction mixture was basified to pH > 12 using 30% NaOH aqueous solution, and then was thoroughly extracted with organic solvent (chloroform for aliphatic amine-containing substrates and ethyl acetate for aminopyridine substrates). The combined organic phase was dried over anhydrous MgSC , filtered, and then concentrated in vacuo. The crude product was analyzed by GC and FontWeight="Bold" FontSize="10" Η NMR, as provided in Table 5.
References:
DOW GLOBAL TECHNOLOGIES LLC;GEORGIA TECH RESEARCH CORPORATION;FISK, Jason S.;GELBAUM, Carol;HOLDEN, Bruce S.;JAGANATHAN, Arvind;LI, Zhao;LIOTTA, Charles;POLLET, Pamela WO2017/112576, 2017, A1 Location in patent:Paragraph 0040-0041
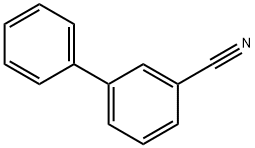
24973-50-0
52 suppliers
$29.00/250mg

177976-49-7
76 suppliers
$55.00/250mg
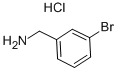
39959-54-1
158 suppliers
$22.00/5g

98-80-6
743 suppliers
$5.00/5g

177976-49-7
76 suppliers
$55.00/250mg
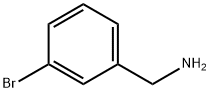
10269-01-9
201 suppliers
$8.00/1g

98-80-6
743 suppliers
$5.00/5g

177976-49-7
76 suppliers
$55.00/250mg
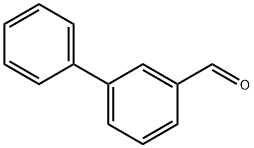
1204-60-0
140 suppliers
$21.00/250mg

177976-49-7
76 suppliers
$55.00/250mg