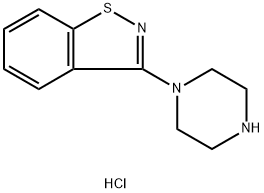
3-Piperazinyl-1,2-benzisothiazole hydrochloride synthesis
- Product Name:3-Piperazinyl-1,2-benzisothiazole hydrochloride
- CAS Number:87691-88-1
- Molecular formula:C11H14ClN3S
- Molecular Weight:255.77

110-85-0
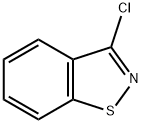
7716-66-7

87691-88-1
General procedure for the synthesis of 3-(piperazin-1-yl)benzo[d]isothiazole hydrochloride from piperazine and 3-chloro-1,2-benzisothiazole: Anhydrous piperazine (49.4 g, 0.57 mol) and tert-butanol (10 ml) were added to a dry, 300 ml round-bottomed flask equipped with a mechanical stirrer, a thermometer, a condenser with nitrogen inlet at the top, and a homogeneous-pressure drip funnel. After purging the flask with nitrogen, it was heated to 100 °C in an oil bath. 3-Chloro-1,2-benzisothiazole (19.45 g, 0.11 mol) was dissolved in tert-butanol (10 mL) and this solution was then added to the addition funnel and slowly added dropwise to the reaction flask over a period of 20 minutes in order to control the exothermic reaction (temperature was maintained at 112-118 °C). After dropwise addition, the yellow solution was heated to reflux (121 °C) and kept at reflux for 24 hours. Completion of the reaction was confirmed by thin layer chromatography. The reaction mixture was cooled to 85 °C and 120 mL of water was added. The turbid solution was filtered and the filter cake was washed with 60 ml of tert-butanol/water (1:1) solution. The filtrate and washings were combined and the pH was adjusted to 12.2 with 50% aqueous sodium hydroxide. the aqueous solution was extracted with toluene (200 mL), the layers were separated and the aqueous layer was then extracted with fresh toluene (100 mL). The toluene layers were combined, washed with 75 mL of water, and then concentrated to 90 mL at 48°C under vacuum. To the concentrate is added isopropanol (210 ml) and the pH is adjusted slowly and dropwise to 3.8 by the addition of 7.6 ml of concentrated hydrochloric acid.The resulting slurry is cooled to 0 °C, stirred for 45 min and filtered. The filter cake was washed with cold isopropanol (50 mL) and then dried under vacuum at 40 °C to give 23.59 g (80% yield) of 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride as an off-white solid.
Yield:87691-88-1 80%
Reaction Conditions:
with hydrogenchloride in water;isopropyl alcohol;toluene;tert-butyl alcohol;
Steps:
3 3-(1-Piperazinyl)-1,2-benzisothiazole · hydrochloride
PREPARATION 3 3-(1-Piperazinyl)-1,2-benzisothiazole · hydrochloride Anhydrous piperazine (49.4 g, 0.57 mol) and t-butanol (10 mL) were added to a dry, 300 mL round bottom flask equipped with a mechanical stirrer, thermometer, condenser topped with a nitrogen inlet, and pressure-equalizing dropping funnel. After the flask was purged with nitrogen, it was heated to 100° C. in an oil bath. A solution of 3-chloro-1,2-benzisothiazole (19.45 g, 0.11 mol) in t-butanol (10 mL) was added to the addition funnel, and then slowly added to the reaction flask over 20 minutes to moderate an exothermic reaction (112-118° C.). Once addition was complete the yellow solution was heated to reflux (121 °C.) and then maintained at reflux for 24 hours. Thin-layer chromatography showed that the reaction was complete. The reaction mixture was cooled to 85° C. and 120 mL of water was added. The hazy solution was filtered and the filter cake rinsed with 60 mL of t-butanol/water (1:1) solution. The pH of the combined filtrate and wash was adjusted to 12.2 with 50% aqueous caustic. The aqueous solution was extracted with toluene (200 mL), the layers were separated, and the aqueous layer was extracted with fresh toluene (100 mL). The combined toluene layers were washed with water (75 mL), and then the toluene solution was concentrated in vacuo at 48° C. to 90 mL. Isopropanol (210 mL) was added to the concentrate and then the pH was slowly adjusted to 3.8 with 7.6 mL of concentrated hydrochloric acid. The resulting slurry was cooled to 0° C., granulated for 45 min, and then filtered. The filter cake was washed with cold isopropanol (50 mL) and then dried in vacuo at 40° C. to afford 23.59 g (80% yield) of 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride as an off white solid.
References:
US5935960,1999,A

110-85-0
589 suppliers
$5.00/5G

7716-66-7
279 suppliers
$32.00/5g
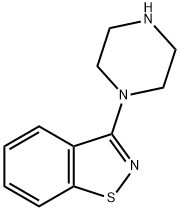
87691-87-0
422 suppliers
$10.00/1g

87691-88-1
192 suppliers
$9.00/1g

110-85-0
589 suppliers
$5.00/5G
33174-74-2
18 suppliers
inquiry
34761-11-0
20 suppliers
inquiry

87691-88-1
192 suppliers
$9.00/1g