
3-Pyridinecarboxamide, 4,6-dichloro-N-methyl- synthesis
- Product Name:3-Pyridinecarboxamide, 4,6-dichloro-N-methyl-
- CAS Number:1609530-95-1
- Molecular formula:C7H6Cl2N2O
- Molecular Weight:205.04
Yield:1609530-95-1 96%
Reaction Conditions:
Stage #1: 4,6-dichloro-nicotinic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 4 h;
Stage #2: methylamine in tetrahydrofuran;dichloromethane at -20 - 0; for 0.75 h;
Steps:
Preparation 1 Synthesis of 4,6-dichloro-N-methyl-pyridine-3-carboxamide
To a stirred solution of 4,6-dichloropyridine-3-carboxylic acid (5000mg, 26.04mmol) in 130 mL of anh. DCM one drop of DMF was added and the temperature was lowered to 0 °C. Oxalyl chloride (6.7mL, 78.12mmol) was added dropwise and the mixture was stirred at room temperature for 4 hr. The solvent was evaporated (co-evaporated with AcOEt) and the crude was redissolved in DCM (66 mL) and cooled to -20 °C. Methylamine (2 M in THF, 32 mL, 64 mmol) was added dropwise and the mixture was stirred at 0 °C for 45 min. Excess water was added followed by NaHC03 sat. solution. The phases were separated and the aqueous phase was extracted with DCM (x 1). The combined organic extracts were dried over anh. MgS04, filtered and evaporated to have the title compound (5.1 lg, 96%) as a white solid. HPLC/MS tR=1.65min (100%). LRMS (m/z): 205,207,209 (2 Cl). ^-RMN (400 MHz, CDC13) d ppm 3.05 (d, J = 4.9 Hz, 3H), 6.22 (brs, 1H), 7.42 (s, 1H), 8.66 (s, 1H).
References:
WO2021/204626,2021,A1 Location in patent:Page/Page column 43-44
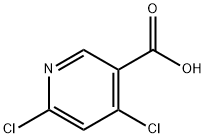
73027-79-9
269 suppliers
$13.00/1g
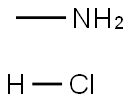
593-51-1
559 suppliers
$21.00/100g

1609530-95-1
9 suppliers
inquiry

593-51-1
559 suppliers
$21.00/100g

107836-75-9
21 suppliers
$134.31/250mgs:

1609530-95-1
9 suppliers
inquiry

73027-79-9
269 suppliers
$13.00/1g

6638-79-5
589 suppliers
$6.00/25g

1609530-95-1
9 suppliers
inquiry
40296-46-6
335 suppliers
$6.00/1g

1609530-95-1
9 suppliers
inquiry
