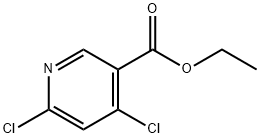
Ethyl 4,6-dichloronicotinate synthesis
- Product Name:Ethyl 4,6-dichloronicotinate
- CAS Number:40296-46-6
- Molecular formula:C8H7Cl2NO2
- Molecular Weight:220.05
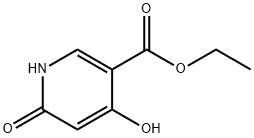
6975-44-6

40296-46-6
The general procedure for the synthesis of ethyl 4,6-dichloronicotinate from ethyl 4,6-dihydroxynicotinate was as follows: ethyl 4,6-dihydroxynicotinate (60 g, 0.328 mol) was slowly added to phosphorus triclosan (POCl3, 500 mL), followed by heating and refluxing for 2 hours. Upon completion of the reaction, the excess phosphorous trichloride was removed by distillation under reduced pressure. The residue was carefully poured into ice water, stirred for 30 min, and then extracted with ethyl acetate (EtOAc, 3 times). The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate (MgSO4) and finally concentrated under vacuum to give ethyl 4,6-dichloronicotinate (65 g, 90% yield). The product was identified by 1H NMR (300 MHz, DMSO-d6): δ 8.80 (s, 1H), 7.95 (s, 1H), 4.34 (q, J=6.9 Hz, 2H), 1.31 (t, J=6.9 Hz, 3H); Mass Spectra (ESI) m/z: 220.1 [M+H]+.
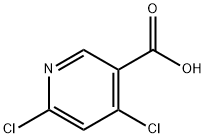
73027-79-9
267 suppliers
$13.00/1g

64-17-5
825 suppliers
$10.00/50g

40296-46-6
324 suppliers
$6.00/1g
Yield:40296-46-6 79%
Reaction Conditions:
Stage #1: 4,6-dichloro-nicotinic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 25; for 2 h;
Stage #2: ethanol in dichloromethane at 25; for 2 h;
Steps:
38.A Step A: ethyl 4,6-dichloronicotinate
A mixture of 4,6-dichloronicotinic acid (20 g, 104 mmol) in DCM (200 mL) was cooled to 0 °C before the addition of DMF (0.807 ml, 10.42 mmol) and oxalyl chloride (10.94 ml, 125 mmol). The resulting reaction was stirred at about 0 °C-25 °C. After about 2h, EtOH (48,7 ml, 833 mmol) was slowly added and stirred at about 25 °C for 2 h. The reaction mixture was diluted with ether (50 mL) and the resulting solution was washed with saturated aqueous NaHC03 (3 x 50 mL). The extracts were combined and dried over anhydrous Na2S04, filtered, and the solvent was removed in vacuo to provide ethyl 4,6-dichloronicotinate (18 g. 79%): LC/MS (Table 1, Method d) Rt=1.91 min.; MS m/z = 220 (M+H).
References:
WO2016/198908,2016,A1 Location in patent:Page/Page column 80; 81

6975-44-6
206 suppliers
$17.67/250mgs:

40296-46-6
324 suppliers
$6.00/1g
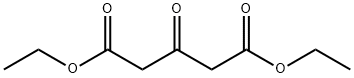
105-50-0
405 suppliers
$6.00/5g

40296-46-6
324 suppliers
$6.00/1g

6975-44-6
206 suppliers
$17.67/250mgs:

40296-46-6
324 suppliers
$6.00/1g

73027-79-9
267 suppliers
$13.00/1g

40296-46-6
324 suppliers
$6.00/1g