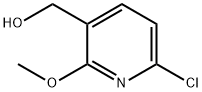
3-Pyridinemethanol, 6-chloro-2-methoxy- synthesis
- Product Name:3-Pyridinemethanol, 6-chloro-2-methoxy-
- CAS Number:1260812-74-5
- Molecular formula:C7H8ClNO2
- Molecular Weight:173.6
38496-18-3
384 suppliers
$6.00/1g

124-41-4
730 suppliers
$12.00/25g

1260812-74-5
17 suppliers
inquiry
1228898-61-0
32 suppliers
$60.00/1g
Yield:-
Reaction Conditions:
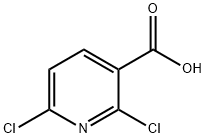
Stage #1:2,6-dichloropyridine-3-carboxylic acid;sodium methylate with methanol at 70; for 5 h;
Stage #2: with borane-THF in tetrahydrofuran at 0 - 20; for 1 h;Overall yield = 20.22 g;
Steps:
5.1.A; 5.1.B
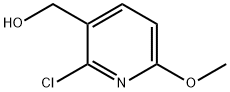
Step A: 2,6-Dichloronicotinic acid (30g, 156.3 mmol) was dissolved in methanol (300 mL) at room temperature. Sodium ethoxide (5.4 M in MeOH, 781.3 mmol, 145 mL) was added slowly at the same temperature. The reaction mixture was stirred at 70 °C for 5 hours and cooled to room temperature. The precipitated solid from reaction mixture was filtered off and the mother liquor was concentrated under vacuum. All solid collected was dissolved in water (300 mL) and the solution was acidified with 6N HC1 solution to pH 6 at 0 °C. The aqueous layer was extracted with ethyl acetate five times. The combined organic layers were dried over MgS04. The organic layer was filtered off and concentrated to give a 1 : 1 mixture of 6-chloro-2- methoxynicotinic acid and 2-chloro-6-methoxynicotinic acid (20.22g, 69%). MS m/z 188 [M+H]+. Step B: The mixture obtained above (11.48g, 61.2 mmol) was dissolved in THF (50 mL) and cooled to 0 °C. Borane-THF complex (1.0 M in THF, 200 mL) was added slowly at 0 °C. The reaction mixture was stirred at room temperature for 1 hour and cooled back to 0 °C. Aqueous 6N HC1 (15 mL) was added very slowly at 0 °C. The aqueous layer was neutralized with a saturated NaHC03 solution to pH 7. Dichloromethane was added and the aqueous layer was extracted. The combined organic layers were dried over K2C03, filtered and concentrated under vacuum to yield 10.6 g of corresponding alcohols. The residue can be used directly in the oxidation step or purified on silica gel with the mixture of ethyl acetate and hexane. TLC Rf 0.31 (30% EtOAc in hexane). MS m/z 174 [M+H]+.
References:
PTC THERAPEUTICS, INC.;F. HOFFMANN-LA ROCHE AG;LEE, Chang-Sun;CHOI, Soongyu;KARP, Gary Mitchell;KOYAMA, Hiroo;RATNI, Hasane WO2013/130689, 2013, A1 Location in patent:Paragraph 00931; 00932; 00933
95652-81-6
94 suppliers
$23.00/100mg

1260812-74-5
17 suppliers
inquiry
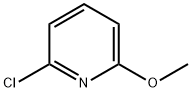
17228-64-7
316 suppliers
$6.00/5g

1260812-74-5
17 suppliers
inquiry