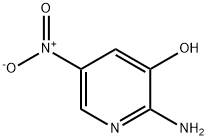
3-Pyridinol, 2-amino-5-nitro- synthesis
- Product Name:3-Pyridinol, 2-amino-5-nitro-
- CAS Number:908248-27-1
- Molecular formula:C5H5N3O3
- Molecular Weight:155.11
![6-Nitrooxazolo[4,5-b]pyridin-2(3H)-one](/CAS/GIF/21594-54-7.gif)
21594-54-7

908248-27-1
The general procedure for the synthesis of 2-amino-5-nitropyridin-3-ol using 6-nitrooxazolo[4,5-b]pyridin-2(3H)-one as starting material was as follows: to a solution of 6-nitrooxazolo[4,5-b]pyridin-2(3H)-one (124 mmol) in ethanol (100 mL), a solution of sodium hydroxide (500 mmol) in water (180 mL) was added. The reaction mixture was heated to reflux at 80°C for 3 hours. Upon completion of the reaction, the reaction was quenched with concentrated hydrochloric acid (40 mL), followed by adjusting the pH with 2 M sodium carbonate solution to 8. The precipitated solid was collected by filtration to afford the target product, 2-amino-5-nitropyridin-3-ol, in 86% yield and the product was a yellow solid.
![6-Nitrooxazolo[4,5-b]pyridin-2(3H)-one](/CAS/GIF/21594-54-7.gif)
21594-54-7
12 suppliers
inquiry

908248-27-1
45 suppliers
$110.00/50mg
Yield:908248-27-1 86%
Reaction Conditions:
Stage #1: 6-nitro-2H,3H-[1,3]oxazolo[4,5-b]pyridin-2-onewith sodium hydroxide;water in ethanol at 80; for 3 h;
Stage #2: with hydrogenchloride;sodium carbonate in ethanol;water; pH=8;
Steps:
30.3
A solution of sodium hydroxide (500 mmol) in water (180 ml) was added to a solution of 6- nitrooxazolo[4,5-b]pyridin-2(3H)-one (124 mmol) in ethanol (100 ml) and the reaction mixture was heated at 80 °C for 3 h. The reaction mixture was quenched with concentrated hydrochloric acid (40 mL) and the pH adjusted to 8 with 2 M sodium carbonate. The precipitated solids were collected by filtration to provide 2-amino-5-nitropyridin-3-ol in 86% yield as a yellow solid.
References:
WO2009/23844,2009,A2 Location in patent:Page/Page column 120-121
896161-12-9
7 suppliers
inquiry

908248-27-1
45 suppliers
$110.00/50mg
16867-03-1
587 suppliers
$10.00/25g

908248-27-1
45 suppliers
$110.00/50mg
![2,3-Dihydropyrido[2,3-d][1,3]oxazol-2-one](/CAS/GIF/60832-72-6.gif)
60832-72-6
208 suppliers
$5.00/1g

908248-27-1
45 suppliers
$110.00/50mg