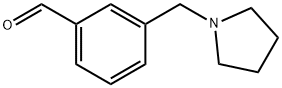
3-(PYRROLIDIN-1-YLMETHYL)BENZALDEHYDE 97 synthesis
- Product Name:3-(PYRROLIDIN-1-YLMETHYL)BENZALDEHYDE 97
- CAS Number:884507-42-0
- Molecular formula:C12H15NO
- Molecular Weight:189.25
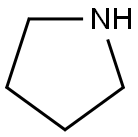
123-75-1
579 suppliers
$10.00/5g
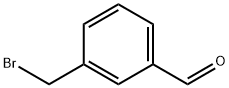
82072-23-9
116 suppliers
$22.00/250mg

884507-42-0
23 suppliers
$45.00/10mg
Yield:884507-42-0 88%
Reaction Conditions:
with potassium carbonate in ethanol at 65; for 1 h;
Steps:
II
Method II: 3-(pyrroldin-1'-yl)-methyl benzaldehyde: A suspension of K2CO3 (2.09 g, 15.2 mmol, 3.00 equiv) in absolute ethanol (20 mL) was treated with pyrroldine (439 μL, 5.05 mmol, 1.00 equiv). 3-(bromomethyl)-benzaldehyde (1.00 g, 5.05 mmol, 1.00 equiv) was introduced, and the reaction was heated to 65° C. for 1 h. The reaction was cooled and filtered. The cake was washed with more ethanol. The filtrate was concentrated to a cloudy oil and partitioned between DCM (50 mL) and 2% w/v aq NaHCO3 (50 mL). The organic phase was collected, and the aq layer was extracted with DCM (2×50 mL). All organic layers were combined, dried (Na2SO4), filtered, and concentrated, giving 3-(pyrrolidin-1-ylmethyl)-benzaldehyde (846 mg, 88% yield) as a pale yellow oil, which was used without further purification. 1H-NMR: 300 MHz, (CDCl3) d: 10.00 (s, 1H), 7.84 (s, 1H), 7.76 (d, J=7.6 Hz, 1H), 7.62 (d, J=7.6 Hz, 1H), 7.47 (dd, J=7.6 Hz, 7.6 Hz, 1H), 3.69 (s, 2H), 2.52 (m, 4H), 1.79 (m, 4H). LCMS-ESI+: calc'd for C12H16NO: 190.1 (M+H+); Found: 190.1 (M+H+).
References:
US2010/143301,2010,A1 Location in patent:Page/Page column 44
321198-22-5
19 suppliers
$199.00/1g

884507-42-0
23 suppliers
$45.00/10mg
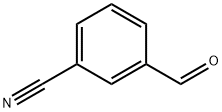
24964-64-5
335 suppliers
$9.00/1g

884507-42-0
23 suppliers
$45.00/10mg
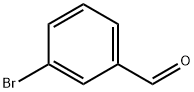
3132-99-8
499 suppliers
$5.00/10g

884507-42-0
23 suppliers
$45.00/10mg