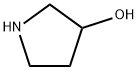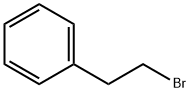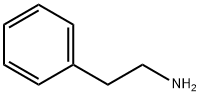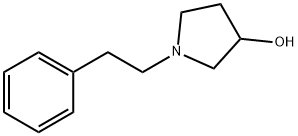
3-Pyrrolidinol, 1-(2-phenylethyl)- synthesis
- Product Name:3-Pyrrolidinol, 1-(2-phenylethyl)-
- CAS Number:79278-81-2
- Molecular formula:C12H17NO
- Molecular Weight:191.27
Yield:79278-81-2 61%
Reaction Conditions:
with potassium carbonate in toluene at 110;
Steps:
4.1.3. 5-(2-((1-Phenethylpyrrolidin-3-yl)oxy)phenyl)-3-(pyridin-4-yl)-1,2,4-oxadiazole (1)
A mixture of pyrrolidin-3-ol (0.100 g, 1.11 mmol, 1 eq), (2-bromoethyl) benzene (0.307mL, 2.23 mmol, 2 eq) and K2CO3 (0.769 g, 5.57 mmol, 5 eq) in toluene (5 mL) was heatedat 110 °C overnight. The reaction mixture was concentrated under reduced pressure. Theresidue was taken up with 1N HCl and extracted with CH2Cl2 twice. The aqueous phasewas basified by the addition of K2CO3 and extracted with CH2Cl2. The organic phase wasdried over MgSO4, filtered, and concentrated under reduced pressure to afford 0.130 g(61%) of 1-(2-phenylethyl)pyrrolidin-3-ol as a white solid. LC-MS (ESI, m/z): 192 [M + H]+.1H NMR (400 MHz, DMSO-d6) d ppm 7.12-7.33 (m, 5H), 4.67 (d, J = 4.6 Hz, 1H), 4.12-4.23(m, 1H), 2.65-2.80 (m, 3H), 2.55-2.64 (m, 3H), 2.41-2.48 (m, 1H), 2.34 (m, 1H), 1.90-2.02 (m,1H), 1.45-1.58 (m, 1H).
References:
Ahmad, Shamshad;Arzel, Philippe;Bardiot, Dorothée;Canard, Bruno;Castermans, Karolien;Chaltin, Patrick;De Jonghe, Steven;Decroly, Etienne;Delpal, Adrien;Eydoux, Cecilia;Guillemot, Jean-Claude;Hilgenfeld, Rolf;Jochmans, Dirk;Klaassen, Hugo;Koukni, Mohamed;Lescrinier, Eveline;Leyssen, Pieter;Lyoo, Heyrhyoung;Marchand, Arnaud;Neyts, Johan;Robinson, Colin;Snijder, Eric J.;Sun, Xinyuanyuan;Vangeel, Laura;Wanningen, Patrick;Zhang, Linlin;Zwaagstra, Marleen;van Hemert, Martijn J.;van Kuppeveld, Frank [Molecules,2022,vol. 27,# 3,art. no. 1052]