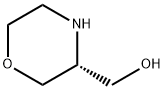
3(R)-HYDROXYMETHYLMORPHOLINE synthesis
- Product Name:3(R)-HYDROXYMETHYLMORPHOLINE
- CAS Number:211053-49-5
- Molecular formula:C5H11NO2
- Molecular Weight:117.15
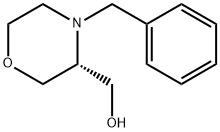
101376-26-5

211053-49-5
General procedure for the synthesis of (R)-3-hydroxymethylmorpholine from (R)-(4-benzyl-3-morpholinyl)-methanol: (a) Preparation of (3R)-morpholin-3-ylmethanol: [(3R)-4-benzylmorpholin-3-yl]methanol (cf. J. Med. Chem.; 29; 1986; 1288-1290; 1.1 g, 5.4 mmol) was dissolved in ethanol (25 mL), palladium hydroxide (20% loaded on activated carbon, 0.7 g) and acetic acid (0.5 mL). The reaction mixture was stirred overnight at room temperature under hydrogen atmosphere (1.2 bar). After completion of the reaction, the catalyst was removed by filtration and the solvent was removed by evaporation under reduced pressure. The residue except 200 mg was dissolved in ether (1 mL) and tetrahydrofuran (10 mL). The solution was purified by passing it through a strong cation exchange column (Isolute SCX-2, 10 g). The column was first washed with tetrahydrofuran, followed by elution of the target product with ammonia-saturated methanol. The solvent was removed by evaporation under reduced pressure to give 0.3625 g (57% yield) of (3R)-morpholin-3-ylmethanol as an oil.1H NMR (500 MHz, CD3OD): δ 2.9 (m, 3H), 3.3 (t, 1H), 3.5 (m, 3H), 3.7-3.9 (m, 2H).
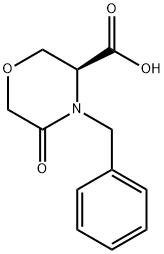
106973-37-9
60 suppliers
inquiry

211053-49-5
112 suppliers
$53.00/100mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: dimethylsulfide borane complex; triethylamine / tetrahydrofuran / 5 h / 0 - 65 °C
2: hydrogen; palladium 10% on activated carbon / methanol / 48 h / 2100.21 Torr
References:
Borsari, Chiara;Rageot, Denise;Dall'Asen, Alix;Bohnacker, Thomas;Melone, Anna;Sele, Alexander M.;Jackson, Eileen;Langlois, Jean-Baptiste;Beaufils, Florent;Hebeisen, Paul;Fabbro, Doriano;Hillmann, Petra;Wymann, Matthias P. [Journal of Medicinal Chemistry,2019,vol. 62,# 18,p. 8609 - 8630]

101376-26-5
97 suppliers
$23.00/100mg

211053-49-5
112 suppliers
$53.00/100mg