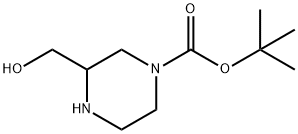
1-Boc-3-hydroxymethyl-piperazine synthesis
- Product Name:1-Boc-3-hydroxymethyl-piperazine
- CAS Number:301673-16-5
- Molecular formula:C10H20N2O3
- Molecular Weight:216.28
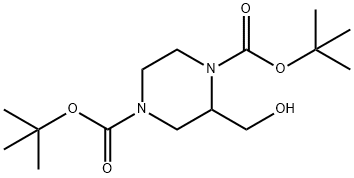
143540-05-0

301673-16-5
General procedure for the synthesis of 1-BOC-3-hydroxymethylpiperazine from 1,4-bis(Boc)-2-piperazinemethanol: To a solution of di-tert-butyl 2-(hydroxymethyl)piperazine-1,4-dicarboxylate (4.00 g, 12.6 mmol) in ethanol (110 ml) was added sodium hydroxide (1.99 g, 49.8 mmol), and the mixture was heated under reflux conditions for 16 hours. After completion of the reaction, the solvent was removed by evaporation under reduced pressure. The residue was poured into water and the mixture was extracted with ethyl acetate and the organic phase was dried with anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to afford the target product 1-BOC-3-hydroxymethylpiperazine (2.71 g, 99.6% yield) as a solid. The structure of the product was confirmed by 1H-NMR (CDCl3): δ 1.46 (9H, s), 2.12 (1H, br s), 2.64-3.01 (6H, br s), 3.47-3.53 (1H, m), 3.62-3.67 (1H, m), 3.89 (2H, br s).
129799-08-2
173 suppliers
$11.00/250mg

301673-16-5
177 suppliers
$15.00/250mg
Yield:301673-16-5 83%
Reaction Conditions:
with sodium tetrahydroborate;ethanol;nickel dichloride at 0; for 4 h;Inert atmosphere;
Steps:
125.1
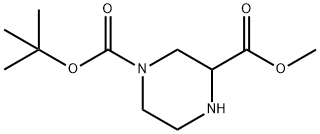
To a mixture of 1-tert-butyl 3-methyl piperazine-1, 3-dicarboxylate (1.0 g, 4.1 mmol) , nickel chloride (640 mg, 4.9 mmol) and anhydrous ethanol (10 mL) was added sodium borohydride (380 mg, 10.0 mmol) at 0 . The mixture was stirred for 4 h and quenched with ice-water (5 mL) . The mixture was adjusted with concentrated hydrochloric acid to pH 1. After the mixture was clear, a NaOH aqueous solution (1.0 M) was added to adjust pH 10. The resulting mixture was extracted with EtOAc (10 mL × 3) . The combined organic layers were dried over anhydrous Na2SO4 and concentrated to give tert-butyl 3- (hydroxymethyl) piperazine-1-carboxylate as a reseda solid (890 mg, 83) .1H NMR (400 MHz, CD3OD) : δ ppm 3.99-4.02 (m, 1H) , 3.90-3.93 (m, 1H) , 3.48-3.54 (m, 2H) , 2.96-3.00 (m, 1H) , 2.83-2.93 (m, 1H) , 2.66-2.72 (m, 2H) , 2.56-2.66 (m, 1H) , 1.48 (m, 9H) and MS-ESI: m/z 217.10 [M+H] +.
References:
SUNSHINE LAKE PHARMA CO., LTD.;ZHANG, Yingjun;LIU, Bing;YU, Tianzhu;ZHANG, Xiangyu;ZHANG, Shiguo;ZHANG, Jiancun;CHENG, Changchung WO2016/34134, 2016, A1 Location in patent:Paragraph 00686

143540-05-0
65 suppliers
$48.00/250mg

301673-16-5
177 suppliers
$15.00/250mg
![TERT-BUTYL 3-OXOTETRAHYDRO-1H-OXAZOLO[3,4-A]PYRAZINE-7(3H)-CARBOXYLATE](/CAS2/GIF/935544-47-1.gif)
935544-47-1
29 suppliers
$1103.00/1g

301673-16-5
177 suppliers
$15.00/250mg