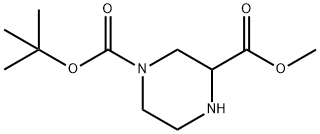
METHYL 4-BOC-PIPERAZINE-2-CARBOXYLATE synthesis
- Product Name:METHYL 4-BOC-PIPERAZINE-2-CARBOXYLATE
- CAS Number:129799-08-2
- Molecular formula:C11H20N2O4
- Molecular Weight:244.29
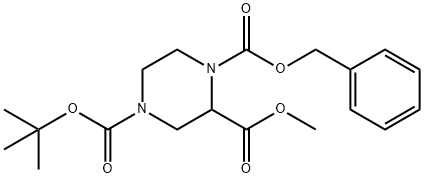
126937-42-6

129799-08-2
Step 3: 10% Pd-C (0.2 equiv) was added to a stirred methanol solution of 0.1 M methyl 1-Cbz-4-Boc-2-piperazinecarboxylate (A2) and the mixture was stirred for 3 hours at room temperature under hydrogen atmosphere. After completion of the reaction, the mixture was filtered, the filter cake was washed with methanol and the filtrate was evaporated under reduced pressure to give methyl 1-BOC-3-piperazinecarboxylate (A3) in 95% yield. The product was characterized as follows: 1H NMR (400 MHz, CDCl3, 300 K) δ 4.02 (1H, m), 3.74 (3H, s), 3.70 (1H, m), 3.43 (1H, m), 3.20 (1H, m), 3.04 (2H, m), 2.75 (1H, m), 2.14 (1H, m), 1.47 (9H, s). s).MS (ES+) C11H20N2O4 theoretical value 244, measured value: 267 (M + Na)+.

67-56-1
790 suppliers
$7.29/5ml-f
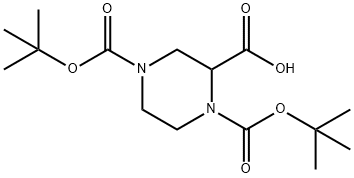
181955-79-3
115 suppliers
$52.00/5g

129799-08-2
173 suppliers
$11.00/250mg
Yield:129799-08-2 85.2%
Reaction Conditions:
Stage #1:N,N'-di-tert-butyloxycarbonylpiperazine-2-carboxylic acid with pyridine;oxalyl dichloride in tetrahydrofuran at 10 - 30;
Stage #2:methanol in tetrahydrofuran at 40 - 50;Reagent/catalyst;
Steps:
3; 4 Synthesis of methyl 4-Boc piperazine-2-carboxylate
In a 1.0L reaction flask, add 1,4-bisBoc piperazine-2-carboxylic acid (20g), add anhydrous THF (200mL), stir well, add pyridine (7.2g), reduce the temperature to 0-10, add dropwise a solution of oxalyl chloride (9.2g) in THF (100mL), control the temperature not to exceed 10, stir at 20-30 for 2-3h, and finally add methanol ( 200mL) continue the reaction at 40-50°C for 2-3h. After the reaction is complete, the reaction solution is concentrated under reduced pressure to remove methanol and pyridine, and ethyl acetate is added to dissolve it. The pH value is adjusted to 9-10 with saturated sodium carbonate aqueous solution. The phases were continuously extracted with EA, the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and column chromatography to obtain the target product (12.6 g, molar yield: 85.2%).
References:
CN112321538, 2021, A Location in patent:Paragraph 0026-0031

126937-42-6
49 suppliers
$45.00/50mg

129799-08-2
173 suppliers
$11.00/250mg

24424-99-5
869 suppliers
$13.50/25G
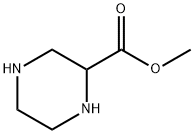
2758-98-7
48 suppliers
inquiry

129799-08-2
173 suppliers
$11.00/250mg

24424-99-5
869 suppliers
$13.50/25G
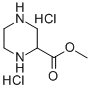
122323-88-0
91 suppliers
$16.00/250mg

129799-08-2
173 suppliers
$11.00/250mg
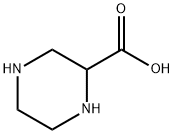
2762-32-5
162 suppliers
$6.00/1g

129799-08-2
173 suppliers
$11.00/250mg