
2-amino-7,7-dimethyl-5-oxo-4-[4-(propan-2-yl)phenyl]-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile synthesis
- Product Name:2-amino-7,7-dimethyl-5-oxo-4-[4-(propan-2-yl)phenyl]-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile
- CAS Number:302323-31-5
- Molecular formula:C21H24N2O2
- Molecular Weight:336.43
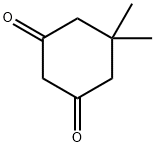
126-81-8
420 suppliers
$5.00/10g
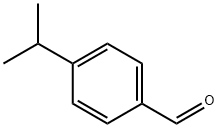
122-03-2
363 suppliers
$6.00/25g

109-77-3
452 suppliers
$10.00/5g
![2-amino-7,7-dimethyl-5-oxo-4-[4-(propan-2-yl)phenyl]-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile](/CAS/20180629/GIF/302323-31-5.gif)
302323-31-5
9 suppliers
inquiry
Yield:302323-31-5 95%
Reaction Conditions:
with ethylenediamine functionalized hyper-crosslinked polyphenanthrene in neat (no solvent) at 20;Green chemistry;
Steps:
2.4. General procedure for the preparation of 2-amino-tetrahydro-4H-chromene and pyran derivatives 1-42
General procedure: p-methoxybenzaldehyde 1{11} (1 mmol), MN 2{1} (1 mmol),dimedone 3{1} (1 mmol), and PPhEDA (10 mg) were mixed at r.t.under solvent-free conditions with stirring. The reaction progress was monitored by TLC (hexane:EA = 6:4). When the reaction was complete, EA (8 mL) was added, and the reaction mixture was centrifuged for 30 min at 6000 rpm. The solvent was decanted and concentrated using a rotary evaporator. The crude product was purified by recrystallization from EtOH (10 mL). This procedure was used for all title compounds. In the re-usability tests, PPhEDA was washed with EA (2 × 8 mL) and dried under vacuum at 80C for 1 h.Detailed spectral data for all the compounds, 1H and13C NMR are given in the Supporting Information (SI).
References:
Kalla, Reddi Mohan Naidu;Varyambath, Anuraj;Kim, Mi Ri;Kim, Il [Applied Catalysis A: General,2017,vol. 538,p. 9 - 18]