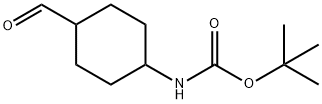
(4-Formylcyclohexyl)-carbamic acid tert-butyl ester synthesis
- Product Name:(4-Formylcyclohexyl)-carbamic acid tert-butyl ester
- CAS Number:304873-80-1
- Molecular formula:C12H21NO3
- Molecular Weight:227.3
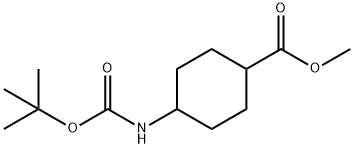
503817-57-0
10 suppliers
inquiry

304873-80-1
11 suppliers
inquiry
Yield:304873-80-1 4 g
Reaction Conditions:
with diisobutylaluminium hydride in tetrahydrofuran;toluene at -78; for 2.25 h;Inert atmosphere;
Steps:
6 Synthesis of tert-butyl (4-formylcyclohexyl) carbamate (45):
To a stirring solution of compound 44 (7 g, 25.88 mmol) in dry THF (100 mL) under argon atmosphere was added diisobutylaluminium hydride (1 M sol. in Toluene, 38.75 mL, 38.75 mmol) dropwise for 15 mm at -78 °C and stirred at the same temperature for 2 h. Thereaction was monitored by TLC; after completion of the reaction, the reaction mixture was quenched with MeOH (10 mL) at -78 °C and stirred for 30 mm and added saturated sodium potassium tartrate solution (50 mL) for 1 h. The organic layer was separated and the aqueous layer was extracted with diethyl ether (2 x 100 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo to obtain the crude. The crude waspurified through silica gel column chromatography using 20% EtOAc/ hexanes to afford compound 45 (4 g, 64%) as colorless liquid. TLC: 30% EtOAc/ hexanes (Rf: 0.5).
References:
WO2018/53157,2018,A1 Location in patent:Page/Page column 63; 64
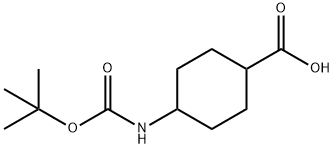
130309-46-5
84 suppliers
$9.00/250mg

304873-80-1
11 suppliers
inquiry

24424-99-5
864 suppliers
$13.50/25G

304873-80-1
11 suppliers
inquiry
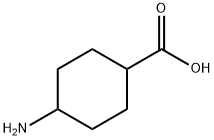
1776-53-0
153 suppliers
$9.00/250mg

304873-80-1
11 suppliers
inquiry
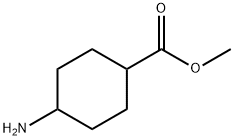
175867-59-1
34 suppliers
$78.00/1g

304873-80-1
11 suppliers
inquiry