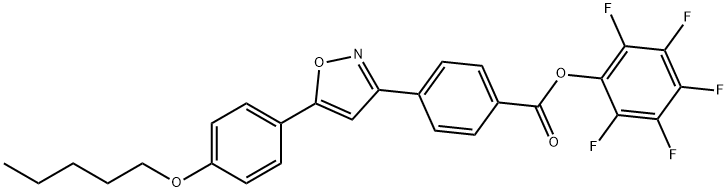
Benzoic acid, 4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]-, 2,3,4,5,6-pentafluorophenyl ester synthesis
- Product Name:Benzoic acid, 4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]-, 2,3,4,5,6-pentafluorophenyl ester
- CAS Number:310459-15-5
- Molecular formula:C27H20F5NO4
- Molecular Weight:517.44
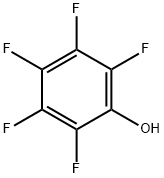
771-61-9
519 suppliers
$6.00/5g
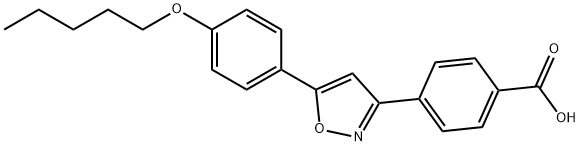
179162-55-1
169 suppliers
$19.00/250mg
![Benzoic acid, 4-[5-[4-(pentyloxy)phenyl]-3-isoxazolyl]-, 2,3,4,5,6-pentafluorophenyl ester](/CAS/20210111/GIF/310459-15-5.gif)
310459-15-5
0 suppliers
inquiry
Yield:310459-15-5 71%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 1.5 h;
Steps:
3" Example 3": Process for the preparation of a compound of formula (lc") (PPIB-PFP).
Example 3": Process for the preparation of a compound of formula (lc") (PPIB-PFP). A suspension of PPIB (1.00 g, 2.85 mmol), EDCI (600 mg, 3.13 mmol) and pentafluorophenol (580 mg, 3.15 mmol) in DMF (20 mL) was stirred at room temperature for 1.5 hours. The precipitate was filtered and dried. Yield: 1.05 g PPIB-PFP, = 71 %. Melting point: 130 °C - 132 °C. White solid.1H NMR (300 MHz, CDCI3): ? = 8.30 (d, J = 8.5 Hz, 2H), 8.04 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 8.8 Hz, 2H), 7.00 (d, J = 8.9 Hz, 2H), 6.78 (s, 1 H), 4.02 (t, J = 6.5 Hz, 2H), 1.82 (m, 2H), 1 .34 - 1.51 (m, 4H), 0.94 (t, J = 7.0 Hz, 3H).
References:
WO2013/34670,2013,A1 Location in patent:Page/Page column 43