
4-(4-CHLORO-6-[(FURAN-2-YLMETHYL)-AMINO]-[1,3,5]TRIAZIN-2-YLAMINO)-PHENOL synthesis
- Product Name:4-(4-CHLORO-6-[(FURAN-2-YLMETHYL)-AMINO]-[1,3,5]TRIAZIN-2-YLAMINO)-PHENOL
- CAS Number:311788-82-6
- Molecular formula:C14H12ClN5O2
- Molecular Weight:317.73
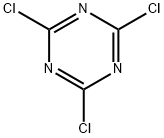
108-77-0
394 suppliers
$13.00/25g
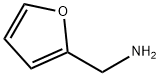
617-89-0
264 suppliers
$16.00/25g
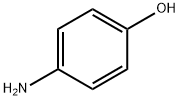
123-30-8
594 suppliers
$10.00/5g
![4-(4-CHLORO-6-[(FURAN-2-YLMETHYL)-AMINO]-[1,3,5]TRIAZIN-2-YLAMINO)-PHENOL](/StructureFile/ChemBookStructure4/GIF/CB6464721.gif)
311788-82-6
9 suppliers
$300.00/1g
Yield:311788-82-6 43%
Reaction Conditions:
Stage #1: 1,3,5-trichloro-2,4,6-triazine;furan-2-ylmethanaminewith N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -20 - 0;
Stage #2: 4-amino-phenolwith N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 0 - 20;
Steps:
2.45
45. 2-(4-Hydroxy-phenylamino)-4-Chloro-6-[(furan-2-ylmethyl)-amino]-[1,3,5]triazine; [0360] To a cooled to -20°c solution of cyanuric chloride (18.44 g, 100 mmol) in anhydrous THF (200 mL) a solution of N,N-diisopropylethylamine (13.57 g, 105 mmol) and furfurylamine (9.71 g, 100 mmol) in anhydrous THF (150 mL) was added dropwise at -20°c for a period of 30 minutes. The resulting mixture was stirred at -20°c for 1 hour (TLC control). Then the reaction mixture was allowed to warm up to 0°c. A solution of N1N- diisopropylethylamine (13.57 g, 105 mmol) and p-aminophenol (10.91 g, 100 mmol) in anhydrous THF (200 mL) was added dropwise at 0°c to the reaction mixture. The resulting mixture was stirred at 0°c for 2 hours then at room temperature for 10 hours. The solvent was removed at reduced pressure. The crude product was purified by column chromatography on silica gel (methanol/ethyl acetate/hexane) giving 1-45 (13.66 g, 43%). 1H-NMR (400MHz, DMSO-D6) δH: 4.46 (2H, d, J=7.5 Hz); 6.24 (1 H, d, J= 7.5 Hz, broad), 6.38 (1 H, broad), 6.70 (2H, d, J= 8.6 Hz), 7.40 (2H, broad), 7.54 (1 H, d, J=1.8 Hz), 8.25- 8.40 (1 H, broad, Z/E forms), 9.20 (1H, broad), 9.50-9.70 (1 H, broad, Z/E forms). LCMS tR (min) 1.56. MS (APCI), m/z 317.52, 319.52 [M+H]+. Mp 188-190°C.
References:
WO2009/91388,2009,A2 Location in patent:Page/Page column 105