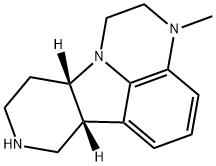
(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline synthesis
- Product Name:(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline
- CAS Number:313368-85-3
- Molecular formula:C14H19N3
- Molecular Weight:229.32
![(6bR,10aS)-Ethyl 3-methyl-2,3,6b,7,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(9H)-carboxylate](/CAS/20180601/GIF/313369-26-5.gif)
313369-26-5
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
Example 8: Preparation of (6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4': 4,5]pyrrolo[1,2,3-de]quinoxaline: tert-butyl (6bR,10aS)-3-methyl-2,3,6b,7,10,10a-hexahydro-1H-pyrido[3',4'. 4,5]pyrrolo[1,2,3-de]quinoxaline-8-carboxylic acid tert-butyl ester (ca. 18.5 g, 57 mmol), KOH (12.7 g, 226 mmol), and n-butanol were added to a 300 mL pressurized flask and heated at 120 °C in an oil bath for 3 hours. Upon completion of the reaction, n-butanol was removed under reduced pressure and 300 mL of water was added, followed by extraction with dichloromethane (DCM). The DCM layers were combined, washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give (6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline. Alternative method: tert-butyl (6bR,10aS)-3-methyl-2,3,6b,7,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8-carboxylate and 1090 mL of HCl were added to a 5L three-necked, round-bottomed flask. the resultant solution was heated at 95°C for 15 hours. Upon completion of the reaction, the reaction mixture was cooled to about 50 °C in an ice bath, followed by the addition of 1090 mL of methyl tert-butyl ether (MTBE). A 25% NaOH solution (1308 mL) was slowly added through the addition funnel, keeping the internal temperature below 30°C. After addition of the NaOH solution, the aqueous layer was pH > 14. 1090 mL of ethyl acetate (EtOAc) was added and the resulting dark-colored mixture was stirred in an ice bath for about 1 hour. The layers were separated and the aqueous layer was extracted with 1090mL EtOAc. The organic layers were combined, washed with 1090 mL of brine, filtered, and concentrated under reduced pressure to give 166.8 g of dark brown liquid (theoretical yield 158.5 g).HPLC analysis showed the product to be 88.1% pure.1H NMR analysis conformed to the expected structure, showing the presence of more than 5% of a single impurity.LC-MS analysis showed 93% of the main peak m/e=230 (M+1). The product was stored in a cold room under N2 protection.1H NMR (CDCl3,300MHz) δ 1.71-1.97 (m,2H), 2.58-2.70 (m,1H), 2.80-2.92 (m,6H), 2.98-3.12 (m,2H), 3.26-3.37 (m,3H), 3.55-3.44 (m,1H) , 6.41 (d,J=7.8Hz,1H), 6.51 (d,J=7.2Hz,1H), 6.65 (t,J=7.8Hz,1H).
![(6bR,10aS)-Ethyl 3-methyl-2,3,6b,7,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(9H)-carboxylate](/CAS/20180601/GIF/313369-26-5.gif)
313369-26-5
51 suppliers
inquiry
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
72 suppliers
inquiry
Yield:313368-85-3 88.1 %Chromat.
Reaction Conditions:
Stage #1: (6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3‘,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline-8-carboxylatewith hydrogenchloride;water at 95; for 15 h;
Stage #2: with sodium hydroxide in tert-butyl methyl ether;water at 10 - 30; pH=> 14;Product distribution / selectivity;
Steps:
8
Example 8: Production of (6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-lH- pyrido-[3',4':4,5]-pyrrolo[l,2,3-de]quinoxaline; (6bR, 10aS)-3-methyl-2,3 ,6b,9, 10, 1 Oa-hexahydro- 1 H-pyrido-[3 ' ,4' :4,5]- pyrrolo[l,2,3-de]quinoxaline-8-carboxylate (ca. 18.5 g, 57mmol), KOH (12.7g, 226mmol) and n-butanol are placed in a 300 ml pressure bottle and heated in an oil bath at 1200C for 3 hours, n-butanol is removed in vacuo and 300 ml of water is added and then extracted with DCM. The DCM layers are combined and washed with brine and dried (Na2SO4). Evaporation of the solvent gives (6bR, 10aS)-ethyl -3 -methyl - 2,3,6b,7,8,9,10,10a-octahydro-lH-pyrido-[3',4':4,5]-pyrrolo[l,2,3-de]quinoxaline.[0094] Alternatively, to a 5-L, 3-necked, round bottomed flask, and the remaining Int-7 is dissolved crude in cone. HCl (1090 mL) before it is added (6bR, 10aS)-3-methyl-2,3,6b,9, 10, 1 Oa-hexahydro- 1 H-pyrido-[3 ',4' :4,5]-pyrrolo[l ,2,3- de]quinoxaline-8-carboxylate to the 5 L reaction vessel. The resultant solution is heated at 95 0C for 15 h. The batch is then cooled in an ice bath to ca. 10 0C, and is added MTBE (1090 mL). To the batch is then added 25% NaOH solution (1308 mL) slowly through an addition funnel while maintaining the internal temperature below 30 0C. The aqueous layer shows pH > 14 after the addition of NaOH solution. To the batch is then added EtOAc (1090 mL), and the resultant dark mixture is stirred in ice bath for ca. 5 min. Layers are separated, and the aqueous layer is extracted with EtOAc (1090 mL). The combined organic layers are washed with brined (1090 mL), filtered, and concentrated under reduced pressure to afford 166.8 g of a dark brown liquid (theoretical yield 158.5 g). HPLC analysis of the liquid showed 88.1% of the desired product. 1H NMR of the product conforms and shows no single impurity over 5%. LC- MS analysis shows ca. 93% of a major peak with M/e = 230 (M+ 1). The product is stored under N2 in the cold room. 1H NMR (CDCl3, 300 MHz) δ 1.71-1.97 (m, 2 H), 2.58-2.70 (m, 1 H), 2.80-2.92 (m, 6 H), 2.98-3.12 (m, 2 H), 3.26-3.37 (m, 3 H), 3.55- 3.64 (m, 1 H), 6.41 (d, J = 7.8 Hz, 1 H), 6.51 (d, J= 7.2 Hz, 1 H), 6.65 (t, J= 7.8 Hz, 1 H).
References:
WO2008/112280,2008,A1 Location in patent:Page/Page column 92-93
![(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole](/CAS/20200119/GIF/1059630-07-7.gif)
1059630-07-7
62 suppliers
inquiry
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
72 suppliers
inquiry
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
63 suppliers
$38.00/100mg
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
72 suppliers
inquiry
![1H-Pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline, 2,3,6b,7,8,9,10,10a-octahydro-3-methyl-](/CAS/20200611/GIF/313369-67-4.gif)
313369-67-4
1 suppliers
inquiry
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
72 suppliers
inquiry
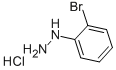
50709-33-6
292 suppliers
$5.00/1g
![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)
313368-85-3
72 suppliers
inquiry