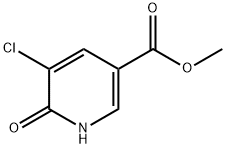
5-Chloro-6-oxo-1,6-dihydro-pyridine-3-carboxylic acid Methyl ester synthesis
- Product Name:5-Chloro-6-oxo-1,6-dihydro-pyridine-3-carboxylic acid Methyl ester
- CAS Number:316166-47-9
- Molecular formula:C7H6ClNO3
- Molecular Weight:187.58

67-56-1
777 suppliers
$9.00/25ml
54127-63-8
197 suppliers
$9.00/1g

316166-47-9
29 suppliers
$60.00/100mg
Yield:316166-47-9 55%
Reaction Conditions:
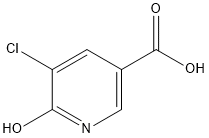
Stage #1: methanol;5-chloro-6-hydroxy-3-pyridinecarboxylic acidwith hydrogenchloride in water; for 5 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water at 20;
Steps:
22.b
b) S-Chloro--hydroxy-nicotinic acid methyl ester; The solution of 2.0 g (11.5 mmol) 3-chloro-2-hydroxypyridine-5-carboxylic acid in 28 mL (35 mmol) hydrochloric acid (1.25M in methanol) was stirred for 5 h under reflux. The reaction was allowed to cool to room temperature, poured on 100 mL 10% aqueous sodium bicarbonate solution and 100 mL ethyl acetate, extracted and washed with 100 mL brine. The aqueous layer was extracted a second time with 100 mL ethyl acetate and the combined organic layers dried with magnesium sulfate, filtered and concentrated under vacuum. The light yellow residue was suspended in 30 mL tert-butyl methyl ether, stirred for 1 h at room temperature and filtered to give the desired compound as a white solid (55%) which was pure enough for the next step. MS (Turbo Spray): m/z = 188.1 [M+H].
References:
WO2010/28981,2010,A1 Location in patent:Page/Page column 59