
methyl(R)-3-(((benzyloxy)carbonyl)amino)-4-hydroxybutanoate synthesis
- Product Name:methyl(R)-3-(((benzyloxy)carbonyl)amino)-4-hydroxybutanoate
- CAS Number:318249-44-4
- Molecular formula:C13H17NO5
- Molecular Weight:267.28
![Benzenesulfonamide, 4-methyl-N-[(3R)-tetrahydro-5-oxo-3-furanyl]-](/CAS/20210305/GIF/162518-06-1.gif)
162518-06-1
0 suppliers
inquiry

318249-44-4
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with trimethylamine in methanol;chloroform;
Steps:
4 Preparation of the Methylester of (R)-4-hydroxy-3-(benzyloxycarbonylamino) Butanoic Acid 5a (Step d)
EXAMPLE 4 Preparation of the Methylester of (R)-4-hydroxy-3-(benzyloxycarbonylamino) Butanoic Acid 5a (Step d) Compound 4 (2.35 g, 10 mmol) (Y=benzyloxycarbonyl, prepared as described in J. Am. Chem. Soc. 1986, 108, 4943-4952) was solubilized in MeOH (15 mL) and added with 18.8 mL (80 mmol) of 25% trimethylamine in MeOH by weight. The reaction was left to stir at room temperature for three days, whereupon CHCl3 was added and the organic phase was washed with HCl 1N and then with NaCl s.s. The organic phase was dried on Na2SO4, filtered and vacuum evaporated to dryness to yield 2.27 g of an oil containing 90% of product (as shown by NMR analysis) and 10% of starting product; 1H NMR (CDCl3): δ 7.35 (s, 5H), 5.45 (br, 1H), 5.10 (s, 2H), 4.08 (m, 1H), 3.75 (d, 2H), 3.65 (s, 3H), 2.65 (d, 2H), 1.60 (brs, 1H). This product was used as such in the following reaction.
References:
US2003/153783,2003,A1

67-56-1
790 suppliers
$9.00/25ml
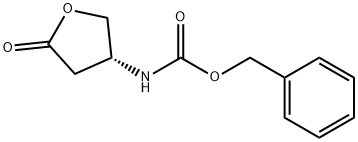
118399-28-3
138 suppliers
$6.00/100mg

318249-44-4
7 suppliers
inquiry
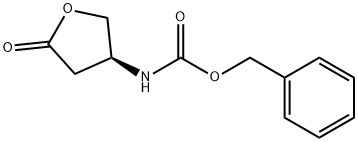
87219-29-2
132 suppliers
$7.00/100mg

318249-44-4
7 suppliers
inquiry
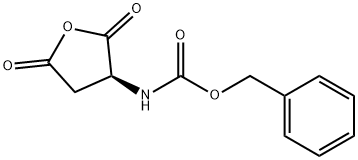
4515-23-5
68 suppliers
$15.00/1g

318249-44-4
7 suppliers
inquiry
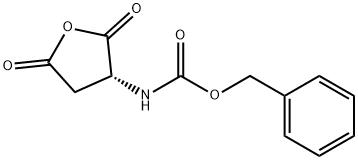
75443-62-8
36 suppliers
$112.00/100mg

318249-44-4
7 suppliers
inquiry