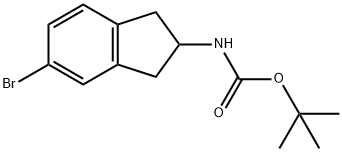
tert-Butyl N-(5-bromo-2,3-dihydro-1H-inden-2-yl)carbamate synthesis
- Product Name:tert-Butyl N-(5-bromo-2,3-dihydro-1H-inden-2-yl)carbamate
- CAS Number:322692-47-7
- Molecular formula:C14H18BrNO2
- Molecular Weight:312.2

24424-99-5
867 suppliers
$13.50/25G
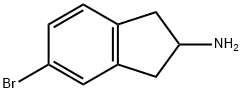
73536-88-6
36 suppliers
inquiry

322692-47-7
13 suppliers
inquiry
Yield:322692-47-7 89%
Reaction Conditions:
with sodium hydrogencarbonate in acetonitrile at 0 - 20;
Steps:
5.4 Step 4) Preparation of 5-bromo-N- (tert-butoxycarbonyl) -2,3-dihydro-1H-inden-2-amine
The title compound can be prepared by the method described in Example 2, Step 4, using 5-bromo-2,3-dihydro-lH-inden-2-amine (1.2 g, 5.7 mmol)Sodium bicarbonate (1.43 g, 17 mmol) and Boc anhydride (2 mL, 8.5 mmol)Suspended in acetonitrile (30 mL)The crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v) = 20/1) to give the title compoundThe product was a white solid (1.6 g, 89%).
A solution of 1-amino-5-bromo-2,3-dihydro-1H-inden-2-ol (1.42 g, 6.23 mmol) and sodium bicarbonate (1.57 g, 18.7 mmol)Was suspended in tetrahydrofuran (60 mL)The mixture was cooled to 0 ° C,To this was added Boc anhydride (1.6 mL, 7.47 mmol).The reaction mixture was stirred at room temperature overnight and concentrated under reduced pressure.The residue was diluted with water (50 mL) and washed with dichloromethane(100 mL x3).The combined organic phases were washed with brine (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure.The residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v) = 3/1)The title compound was obtained as a white solid (2.02 g, 99%).
References:
CN104016979,2017,B Location in patent:Paragraph 0394; 0395; 0396; 0397; 0456;' 0457; 0458; 0459

24424-99-5
867 suppliers
$13.50/25G

322692-47-7
13 suppliers
inquiry
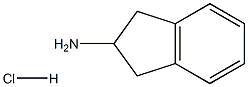
2338-18-3
1 suppliers
$13.43/250mgs:

322692-47-7
13 suppliers
inquiry
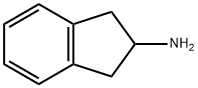
2975-41-9
0 suppliers
$70.00/1g

322692-47-7
13 suppliers
inquiry