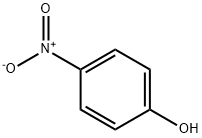
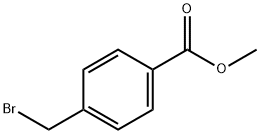
4-(4-Nitro-phenoxymethyl)-benzoic acid methyl ester synthesis
- Product Name:4-(4-Nitro-phenoxymethyl)-benzoic acid methyl ester
- CAS Number:324544-87-8
- Molecular formula:C15H13NO5
- Molecular Weight:287.27
Yield:324544-87-8 96%
Reaction Conditions:
with potassium carbonate in acetone; for 168 h;Reflux;
Steps:
4.2.3. Methyl 4-[(4'-nitrophenoxy)methyl]benzoate
4-Nitrophenol (2.40 g, 17.2 mmol) and methyl 4-bromomethylbenzoate (2.00 g, 8.7 mmol) were dissolved in acetone (150 mL) before adding potassium carbonate (7.0 g). The mixture was refluxed for 7 days under constant stirring. The reaction was monitored by TLC (dichloromethane). At the completion of the reaction acetone was evaporated under reduced pressure and the residue was taken up in a mixture of dichloromethane and water. The crude material was extracted into dichloromethane (3×40 mL) and the combined organic layers were washed with sodium hydroxide (3 M) until the aqueous layer became colourless. The organic layer was dried over anhydrous sodium sulfate, filtered and evaporated to dryness. The crude material was chromatographed (silica gel, hexane/dichloromethane 1:2) to afford methyl 4-[(4'-nitrophenoxy)methyl]benzoate (2.40 g, 96%) as white crystals: mp 161-162 °C; Rf (CH2Cl2) 0.95; IR (neat) νmax 2960, 1707, 1592, 1511, 1498, 1261, 1107 cm-1; 1H NMR (400 MHz, CDCl3) δ 3.93 (s, 3H, OCH3), 5.22 (s, 2H, CH2), 7.03 (d, J 7.1 Hz, 2H, ArH), 7.49 (d, J 8.4 Hz, 2H, ArH), 8.07 (d, J 8.4 Hz, 2H, ArH), 8.20 (d, J 7.1 Hz, 2H, ArH); 13C NMR (100 MHz, CDCl3) δ 51.4, 69.4, 114.4, 114.4, 125.2, 125.3, 126.4, 126.5, 129.3, 140.1, 162.8; Anal. Calcd for C15H13NO5: C 62.72; H 4.56; N 4.88. Found C 62.96; H 4.71; N 4.75%.
References:
Malik, Qasim M.;Ijaz, Sadia;Craig, Donald C.;Try, Andrew C. [Tetrahedron,2011,vol. 67,# 32,p. 5798 - 5805] Location in patent:experimental part