
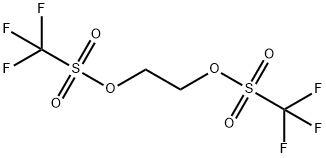
2-(trifluoromethoxy)ethyl trifluoromethanesulfonate synthesis
- Product Name:2-(trifluoromethoxy)ethyl trifluoromethanesulfonate
- CAS Number:329710-76-1
- Molecular formula:C4H4F6O4S
- Molecular Weight:262.13
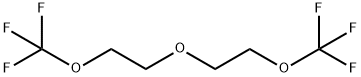
329710-73-8
5 suppliers
inquiry

329710-76-1
13 suppliers
inquiry
Yield: 58%
Reaction Conditions:
with trifluoroacetic acid;trifluoroacetic anhydride at 60; for 120 h;Acidic conditions;
Steps:
195.iib (iib)
A tube was charged with 1-trifluoromethoxy-2- (2-trifluoromethoxy-ethoxy)-ethane (5.81g, 24mmol). Trifluoroacetic acid (0.55 ml, 6. 2MMOL) and trifluoroacetic anhydride (16 ml, 95. 1MMOL) were added, the tube sealed well under N2 atmosphere, and then immersed in a 60°C heated oil bath and heated for 5 days. The tube was removed from the oil bath and cooled to room temperature. The reaction mixture was concentrated in vacuo with ambient temperature bath. The residue was partitioned between dichloromethane and water, the layers were separated, the organic layer was dried over MGS04, and concentrated in vacuo. The crude material was purified by bulb to bulb distillation with mild vacuum at 120°C and trapped AT-78°C to yield (7.26g, 58%) of TRIFLUORO-METHANESULFONIC acid 2-trifluoromethoxy-ethyl ester : IH NMR (400 MHz, CDC13) : 8 = 4.69 (2H, m), 4.27 (2H, m).
References:
ELI LILLY AND COMPANY WO2004/52858, 2004, A2 Location in patent:Page 163
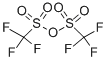
358-23-6
628 suppliers
$10.00/1g

329710-73-8
5 suppliers
inquiry
18928-34-2
23 suppliers
inquiry

329710-76-1
13 suppliers
inquiry
![Pentane, 1-[2-(trifluoromethoxy)ethoxy]-](/CAS/20200515/GIF/329710-75-0.gif)