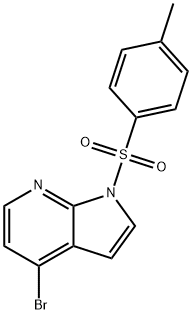
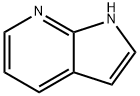
4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE synthesis
- Product Name:4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE
- CAS Number:348640-07-3
- Molecular formula:C14H11BrN2O2S
- Molecular Weight:351.22
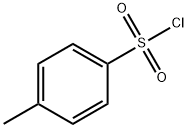
98-59-9
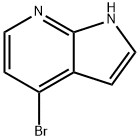
348640-06-2
![4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/348640-07-3.gif)
348640-07-3
4-Bromo-7-azaindole (3 g, 15.22 mmol) was used as starting material and dissolved in tetrahydrofuran (THF, 50 mL) under nitrogen protection in a round bottom flask. The reaction mixture was cooled to 0 °C and sodium hydride (60% dispersed in mineral oil, 0.67 g, 16.75 mmol) was added in batches, and the outgassing phenomenon was observed during the addition. After the addition, the reaction mixture was stirred at room temperature for 30 min. Subsequently, p-toluenesulfonyl chloride (2.14 mL, 16.75 mmol) was slowly added to the reaction system. The reaction mixture was gradually warmed up to room temperature and stirring was continued for 2 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue was dissolved with dichloromethane (DCM, 30 mL). The organic phase was washed with 2 M sodium carbonate solution (2 x 30 mL), dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated to give an orange colored oil. Purification was carried out by fast column chromatography with the eluent ethyl acetate:cyclohexane (1:9, v/v) to afford the target compound 4-bromo-1-[(4-methylphenyl)sulfonyl]-1H-pyrrolo[2,3-B]pyridine as an off-white solid in 92% yield. The product was analyzed by LCMS (Method 5, retention time 5.36 min, molecular ion peak m/z 337 [M + H]+) and 1H NMR (500 MHz, CDCl3) to confirm the structure: δ 8.22 (d, 1H), 8.18 (d, 2H), 7.78 (d, 1H), 7.58 (t, 1H), 7.48 (t, 2H), 7.35 (d , 1H), 6.63 (d, 1H).

98-59-9
625 suppliers
$9.00/5g

348640-06-2
338 suppliers
$6.00/250mg
![4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/348640-07-3.gif)
348640-07-3
113 suppliers
$47.00/1g
Yield:348640-07-3 100%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil;
Steps:
In an ice bath, 4-bromo-7-azaindole (25.5 mmol, 5 g) and p-toluenesulfonyl chloride (38.25 mmol, 7.27 g) were added to a reaction flask, dissolved in 100 mL of anhydrous tetrahydrofuran, 60%NaH (30 mmol, 1.2 g) was slowly added, and the mixture was reacted at room temperature for 6 hours. The reaction was stopped, 50 mL of water was slowly added, the organic solvent was dried by rotary evaporation, extracted with dichloromethane, dried over anhydrous Na2SO4, and separated by column chromatography to afford intermediate a2 (9.0 g, quantitative yield), LC-MS: [M + H] +: 350.
References:
EP3967694,2022,A1 Location in patent:Paragraph 0148
55052-24-9
145 suppliers
$80.00/5G
![4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/348640-07-3.gif)
348640-07-3
113 suppliers
$47.00/1g
271-63-6
573 suppliers
$9.00/5g
![4-BROMO-1-[(4-METHYLPHENYL)SULFONYL]-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/348640-07-3.gif)
348640-07-3
113 suppliers
$47.00/1g