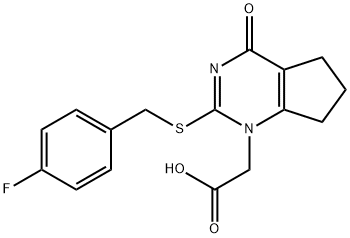
1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo- synthesis
- Product Name:1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-
- CAS Number:356058-42-9
- Molecular formula:C16H15FN2O3S
- Molecular Weight:334.37
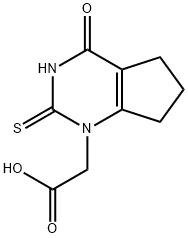
1349902-20-0
6 suppliers
inquiry
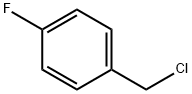
352-11-4
379 suppliers
$5.00/25g
![1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
30 suppliers
$27.00/100mg
Yield:356058-42-9 97%
Reaction Conditions:
Stage #1: 2-(4-oxo-2-thioxo-2,3,4,5,6,7-hexahydro-1H-cyclopenta-[d]-pyrimidin-1-yl) acetic acid;1-chloromethyl-4-fluorobenzenewith sodium carbonate;sodium hydroxide in water;isopropyl alcohol at 40; for 2.5 h;
Stage #2: with formic acid in water;isopropyl alcohol at 20; for 2.5 h;Product distribution / selectivity;
Steps:
7
Example 7 - Alternative method for making (2-{[(4-Fluorophenyl)methyl]thio}-4-oxo-4,5,6,7- tetrahydro-lHcyclopenta[d]pyrimidin-l-yl)acetic acid) (4-Oxo-2-thioxo-2,3,4,5,6,7-hexahydro-lH- cyclopenta [ /]pyrimidin-l-yl)acetic acid (20.0 g, 1.0 eq) was slurried in a mixture of water (112 mL) and isopropyl alcohol (20 mL). NaOH solution (50.9% aqueous, 13.82 g, 1.99 eq) was added followed by a water line wash (10 mL) resulting in a solution. Then Na2C03 (1.50 g, 0.16 eq) was charged and the solution was heated to 40+/-3° C. Thereafter 4-fluorobenzyl chloride (13.4 g, 1.05 eq) was added, followed by a line wash of isopropyl alcohol (12 mL) and the reaction mixture was stirred at 40+/-3° C until the reaction was deemed complete ( ~2.5 hours). The reaction mixture was cooled to 20+/-3° C and formic acid (2.4 g, 0.6 eq) was added resulting in crystallisation of the product within 30 minutes. A second charge of formic acid (6.9 g, 1.7 eq) was added over 1 hour and the slurry was stirred at 20+/-3° C for at least one hour. The slurry was filtered to isolate the product, which was washed twice with a mixture of water (32 mL) and isopropyl alcohol (8 mL), then with isopropyl alcohol (40 mL) and dried in vacuo at 50° C to yield the title compound as an off-white solid (28.6 g, 97%th). 1H NM R (d6 DMSO) δ 1.95 (2H, m), 2.57 (2H, t), 2.85 (2H, t), 4.4 (2H, s), 4.7 (2H, s), 7.15 (2H, dd), 7.45 (2H, dd), ~13.6 (1H, vbrs).
References:
WO2011/146494,2011,A1 Location in patent:Page/Page column 11
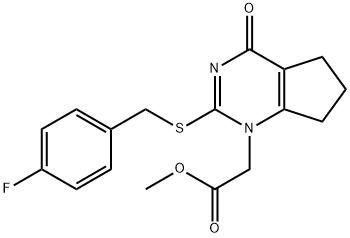
1417526-07-8
1 suppliers
inquiry
![1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
30 suppliers
$27.00/100mg
![4H-CyclopentapyriMidin-4-one, 2-[[(4-fluorophenyl)Methyl]thio]-1,5,6,7-tetrahydro- (9CI)](/CAS/20180709/GIF/451487-18-6.gif)
451487-18-6
8 suppliers
inquiry
![1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
30 suppliers
$27.00/100mg
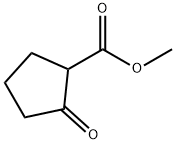
10472-24-9
453 suppliers
$6.00/10g
![1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
30 suppliers
$27.00/100mg
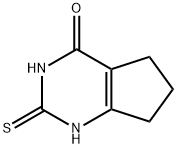
35563-27-0
39 suppliers
$141.00/250mg
![1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
30 suppliers
$27.00/100mg