4-Fluorobenzyl chloride synthesis
- Product Name:4-Fluorobenzyl chloride
- CAS Number:352-11-4
- Molecular formula:C7H6ClF
- Molecular Weight:144.57
459-56-3
304 suppliers
$6.00/5g

352-11-4
389 suppliers
$5.00/25g
Yield:352-11-4 74%
Reaction Conditions:
with 1-pyrrolidinecarboxaldehyde;benzoyl chloride in 1,4-dioxane at 40; for 24 h;Sealed tube;
Steps:
4.4.2.5 Synthesis of 4-Fluorobenzyl chloride (27)
General procedure: 3 1 Following general procedure II (chapter 2.1.2) a solution 4-fluorobenzylic alcoholCl 17 (219 ilL, 253 mg, 2.00 mmol, 1.0 equiv), FPyr (19.7 ilL, 20.4 mg, 0.20 mmol,5 25 10 mol%) in dioxane (1 mL, 2 M) was allowed to react with benzoyl chloride27 (282 ilL, 341 mg, 2.40 mmol, 1.2 equiv) for 24 h at 40 °C. ‘H-N MR of the crudematerial (350 mg) showed full conversion and a ratio chloride 27 to ester 37 of97:3. Chromatographic purification (mass of crude material/5i02 1:14) with Et20/nPen 5:95 delivered chloride 26 in 74% yield (214 mg, 1.48 mmol) as a colorless oil. ‘H- and ‘3C-NMR-data was identicalwith the literature (Klimesova, V.; Koci, J.; Waisser, K.; Kaustova, J.; Möllmann, U. Eur. i. Med. Chem.2009, 44, 2286-2293).M (C7H6CIF) = 144.573 g/mol; rf (5i02, Et20/nPen 95:5) = 0.52; 1H-NMR (400 MHz, CDCI3): 6 [ppm] =7.36 (dd, 2 H, H-3, i = 8.4 Hz, 5.6 Hz), 7.04 (dd, 2 H, H-4, i = 8.4, 8.4 Hz), 4.56 (s, 2 H, H-i); 13C-NMR(100 MHz, CDCI3) 6 [ppm] = 162.6 (d, C-5, i = 243 Hz), 133.4 (C-2), 130.5 (d, C-3, i = 8.6 Hz), 115.7 (d, C-4, i = 22 Hz), 45.5 (C-i); GC-MS (El, 70 eV) m/z [u] = 145 (77, [M+H]j, 144 (100, [M]j, 127 (1, [MF]j, 109 (100, [M-Cl]j, 89 (36), 83 (100), 63 (53), 57 (76), 51 (29).
References:
UNIVERSITAET DES SAARLANDES;HUY, Peter Helmut WO2016/202894, 2016, A1 Location in patent:Page/Page column 96; 97
352-32-9
318 suppliers
$9.00/5g

352-11-4
389 suppliers
$5.00/25g
![Benzene, 1,1'-[oxybis(methylene)]bis[4-fluoro-](/CAS/20210111/GIF/61812-54-2.gif)
61812-54-2
1 suppliers
inquiry

352-11-4
389 suppliers
$5.00/25g
456-22-4
501 suppliers
$10.00/100 g

352-11-4
389 suppliers
$5.00/25g
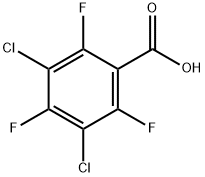
13656-36-5
44 suppliers
$34.00/5g

352-11-4
389 suppliers
$5.00/25g