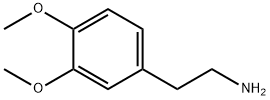
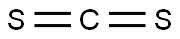3-[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-2-MERCAPTO-3H-QUINAZOLIN-4-ONE synthesis
- Product Name:3-[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-2-MERCAPTO-3H-QUINAZOLIN-4-ONE
- CAS Number:361150-63-2
- Molecular formula:C18H18N2O3S
- Molecular Weight:342.41
118-48-9
435 suppliers
$5.00/5G
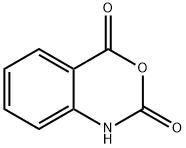
333-20-0
460 suppliers
$13.50/100G
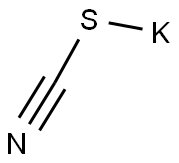
120-20-7
571 suppliers
$5.00/5G
![3-[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-2-MERCAPTO-3H-QUINAZOLIN-4-ONE](/StructureFile/ChemBookStructure7/GIF/CB2333081.gif)
361150-63-2
9 suppliers
inquiry
Yield:361150-63-2 83%
Reaction Conditions:
Stage #1: isatoic anhydride;2-(3,4-dimethoxyphenyl)-ethylamine in water at 20; for 1 h;Green chemistry;
Stage #2: potassium thioacyanatewith acetic acid in water; for 3 h;Heating;Green chemistry;
Steps:
5. General procedure for the preparation of 2-thioxoquinazolinone derivatives 4a-4o
General procedure: A mixture of isatoic anhydride 1 (1 mmol) and the corresponding amine 2 (1 mmol) in H2O (5 mL) was stirred for 1h at ambient temperature. Then, KSCN (1 mmol) in acetic acid (1 mL) and H2O (5 mL) was added to the mixture and the reaction was continued for 3h at 80°C. After completion of the reaction, the crude product was filtered, washed well with water and recrystallized in hot water to gain the pure colorless product.
References:
Rezanejade Bardajee, Ghasem;Ghaedi, Aseyeh;Hekmat, Shohreh;Abarashi, Ghazale;Mahdavi, Mohammad;Akbarzadeh, Tahmineh [Journal of Sulfur Chemistry,2017,vol. 38,# 5,p. 519 - 529]

118-48-9
435 suppliers
$5.00/5G
![3-[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-2-MERCAPTO-3H-QUINAZOLIN-4-ONE](/StructureFile/ChemBookStructure7/GIF/CB2333081.gif)
361150-63-2
9 suppliers
inquiry

120-20-7
571 suppliers
$5.00/5G
![3-[2-(3,4-DIMETHOXY-PHENYL)-ETHYL]-2-MERCAPTO-3H-QUINAZOLIN-4-ONE](/StructureFile/ChemBookStructure7/GIF/CB2333081.gif)
361150-63-2
9 suppliers
inquiry