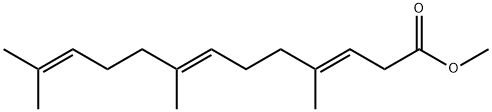
(3E,7E)-4,8,12-Trimethyl-3,7,11-tridecatrienoic acid methyl ester synthesis
- Product Name:(3E,7E)-4,8,12-Trimethyl-3,7,11-tridecatrienoic acid methyl ester
- CAS Number:36237-69-1
- Molecular formula:C17H28O2
- Molecular Weight:264.4

67-56-1
776 suppliers
$9.00/25ml
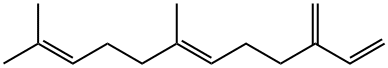
18794-84-8
140 suppliers
$28.00/1G
201230-82-2
1 suppliers
inquiry
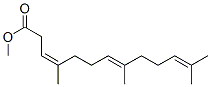
36237-70-4
0 suppliers
inquiry

36237-69-1
0 suppliers
inquiry
Yield:33.333 % de
Reaction Conditions:
with bis-triphenylphosphine-palladium(II) chloride in tetrahydrofuran at 90; under 30003 - 45004.5 Torr; for 64 h;Autoclave;Sealed tube;Overall yield = 61 %; Overall yield = 1.17 g;Reagent/catalyst;Solvent;Pressure;
Steps:
2 Catalytic methoxycarbonylation of 6-farnesene using [PdCl231 complex
In a 75 mL Keim autoclave, containing a stirring bar, were placed (E)-7,11-dimethyl-3- methylenedodeca-1,6,10-triene (1.00 g, 4.89 mmol) in THF (20 ml). Methanol (200 tl, 4.94 mmol) and PdC12(PPh3)2 (80 mg, 0.114 mmol) were added. Then the reactor was sealed and the mixture purged with 3x10 bar of carbon monoxide (CO). While stirring,the autoclave was pressurized to 40 bar with CO and then heated in an oil bath at 90°C.Once the temperature equilibrated to the set point, the autoclave was pressurized to67 bar with CO and the reaction allowed to proceed under these conditions for 64 h.Then the reaction mixture was concentrated on a rotary evaporator to give a cruderesidue which was subsequently distilled on a Kugelrohr apparatus (0.3 mbar/ 170°C) toafford a colorless liquid (1.17 g) containing (3E,7E)-homofarnesic acid methyl ester and(3Z,7E)-homofarnesic acid methyl ester (61 %, 2/1).The desired product (I) obtained had the same spectral characterization as the ones described in the literature for the same product.
References:
WO2014/79691,2014,A1 Location in patent:Page/Page column 9; 10

36237-68-0
0 suppliers
inquiry

36237-69-1
0 suppliers
inquiry
