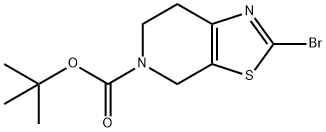
tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate synthesis
- Product Name:tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate
- CAS Number:365996-06-1
- Molecular formula:C11H15BrN2O2S
- Molecular Weight:319.22
![TERT-BUTYL 2-AMINO-6,7-DIHYDROTHIAZOLO[5,4-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/365996-05-0.gif)
365996-05-0
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
Method G - Step e: Synthesis of tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate [0134] To a 100 mL DMF solution containing isoamyl nitrite (8.8 mL, 62.8 mmol) and copper(II) bromide (10.7 g, 48 mmol) was added tert-butyl 2-amino-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate (10 g, 39.2 mmol) at 0°C. The reaction was carried out at 40°C and the reaction mixture was purified. The reaction mixture was stirred at 40°C for 30 min and then concentrated by evaporation. The concentrate was dissolved in 50 mL of water and extracted with dichloromethane (100 mL × 2). The organic phases were combined, washed with brine (30 mL × 2), and dried over anhydrous sodium sulfate. Purification by silica gel column chromatography (eluent: dichloromethane) afforded the target product tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate (5.3 g, 42.4%) as a yellow solid.
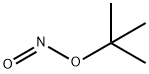
540-80-7
383 suppliers
$29.00/25 g
![TERT-BUTYL 2-AMINO-6,7-DIHYDROTHIAZOLO[5,4-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/365996-05-0.gif)
365996-05-0
158 suppliers
$54.00/1 / G
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
140 suppliers
$56.00/250mg
Yield: 44%
Reaction Conditions:
with copper(ll) bromide in dichloromethane at 0; for 3 h;
Steps:
29.3 tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate
To a stuffing solution of tert-butyl 2-amino-6,7-dihydrothiazolo[5,4-c]pyridine- 5(4H)-carboxylate (2.0 g, 7.84 mmol) in DCM (30 mL) was added tert-butyl nitrite (1.24 g, 12 mmol) and CuBr2 (1.78 g, 8 mmol). The solution was stirred at 0 °C for 3h. Once LCMS showed the reaction to be complete, solvents were then evaporated and the residue was purified with column separation to afford desired product as white solid (1.1 g, yield: 44%);LCMS: 318.9/320.9 (M+1).
References:
EPIZYME, INC.;DUNCAN, Kenneth, W.;CHESWORTH, Richard;MUNCHHOF, Michael, John;SHAPIRO, Gideon WO2015/200677, 2015, A2 Location in patent:Paragraph 00647; 00648
![TERT-BUTYL 2-AMINO-6,7-DIHYDROTHIAZOLO[5,4-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/365996-05-0.gif)
365996-05-0
158 suppliers
$54.00/1 / G
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
140 suppliers
$56.00/250mg
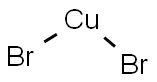
7789-45-9
386 suppliers
$6.00/25g
![TERT-BUTYL 2-AMINO-6,7-DIHYDROTHIAZOLO[5,4-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/365996-05-0.gif)
365996-05-0
158 suppliers
$54.00/1 / G
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
140 suppliers
$56.00/250mg

79099-07-3
0 suppliers
$5.00/5g
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
140 suppliers
$56.00/250mg
188869-05-8
155 suppliers
$8.00/1g
![tert-butyl 2-bromo-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-carboxylate](/CAS/GIF/365996-06-1.gif)
365996-06-1
140 suppliers
$56.00/250mg