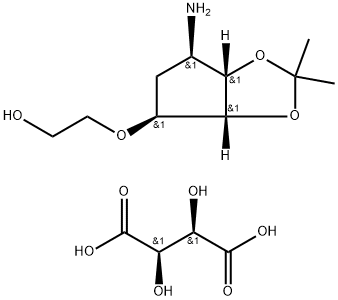
2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid synthesis
- Product Name:2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
- CAS Number:376608-65-0
- Molecular formula:C14H25NO10
- Molecular Weight:367.35
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/NewsImg/2024-03-27/6384712941305119502762577.png)
![N-[(3aS,4R,6S,6aR)-Tetrahydro-6-(2-hydroxyethoxy)-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]carbamic acid phenylmethyl ester](/CAS/GIF/274693-54-8.gif)
274693-54-8
79 suppliers
$20.00/100mg
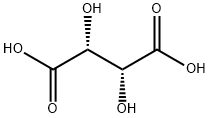
87-69-4
947 suppliers
$5.00/25g
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/CAS/GIF/376608-65-0.gif)
376608-65-0
363 suppliers
$9.00/1g
Yield:376608-65-0 87.47%
Reaction Conditions:
Stage #1: benzyl 6-(2-hydroxyethoxy)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ylcarbamatewith palladium 10% on activated carbon;ammonium formate in methanol at 20 - 50; for 10 h;
Stage #2: L-Tartaric acid at 10 - 50; for 3 h;
Steps:
4-5 Example 5: Preparation of Compound IV
Put 50g of compound III (the preparation method adopts any one of the methods of Example 1 to Example 3) and 250 mL of methanol into a 1L four-necked flask 1.Add 10 g of 10% palladium carbon and 22.4 g of ammonium formate to bottle 1 at 20-30°C, and after the addition, the temperature is raised to 40-50°C and the reaction is stirred for 10 hours, and filtered with suction.The filtrate was transferred to a 1L four-neck flask 2 and the temperature was raised to 40-50°C again, and 21.4g L-tartaric acid was added in batches to 2 while stirring.Then continue to stir the reaction for 1 hour, slowly lower the temperature to 10-20°C and stir the reaction for 2 hours, and a large amount of white solids can be seen to precipitate.Continue to lower the temperature to 0-10°C and react for 0.5h, then suction filter, and wash the filter cake once with 50mL ethanol.The filter cake was dried under reduced pressure at 40° C. for 4 hours to obtain 45.7 g of white powdery solid compound IV with a yield of 87.47% and a GC purity of 99.56% (after free extraction of compound IV with alkali).
References:
CN112724119,2021,A Location in patent:Paragraph 0044-0049
![CarbaMicacid,N-[(3aS,4R,6S,6aR)-tetrahydro-6-hydroxy-2,2-diMethyl-4H-cyclopenta-1,3-dioxol-4-yl]-,phenylMethyl ester](/CAS/GIF/274693-53-7.gif)
274693-53-7
148 suppliers
$5.00/250mg
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/CAS/GIF/376608-65-0.gif)
376608-65-0
363 suppliers
$9.00/1g
![D-erythro-Pent-4-enitol, 1,4,5-trideoxy-2,3-O-(1-methylethylidene)-1-[oxido(phenylmethyl)imino]-, (1Z)-](/CAS/20211123/GIF/345898-95-5.gif)
345898-95-5
3 suppliers
inquiry
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/CAS/GIF/376608-65-0.gif)
376608-65-0
363 suppliers
$9.00/1g
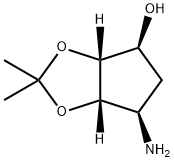
155899-66-4
219 suppliers
$20.00/1g
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/CAS/GIF/376608-65-0.gif)
376608-65-0
363 suppliers
$9.00/1g
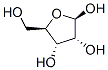
36468-53-8
18 suppliers
inquiry
![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](/CAS/GIF/376608-65-0.gif)
376608-65-0
363 suppliers
$9.00/1g