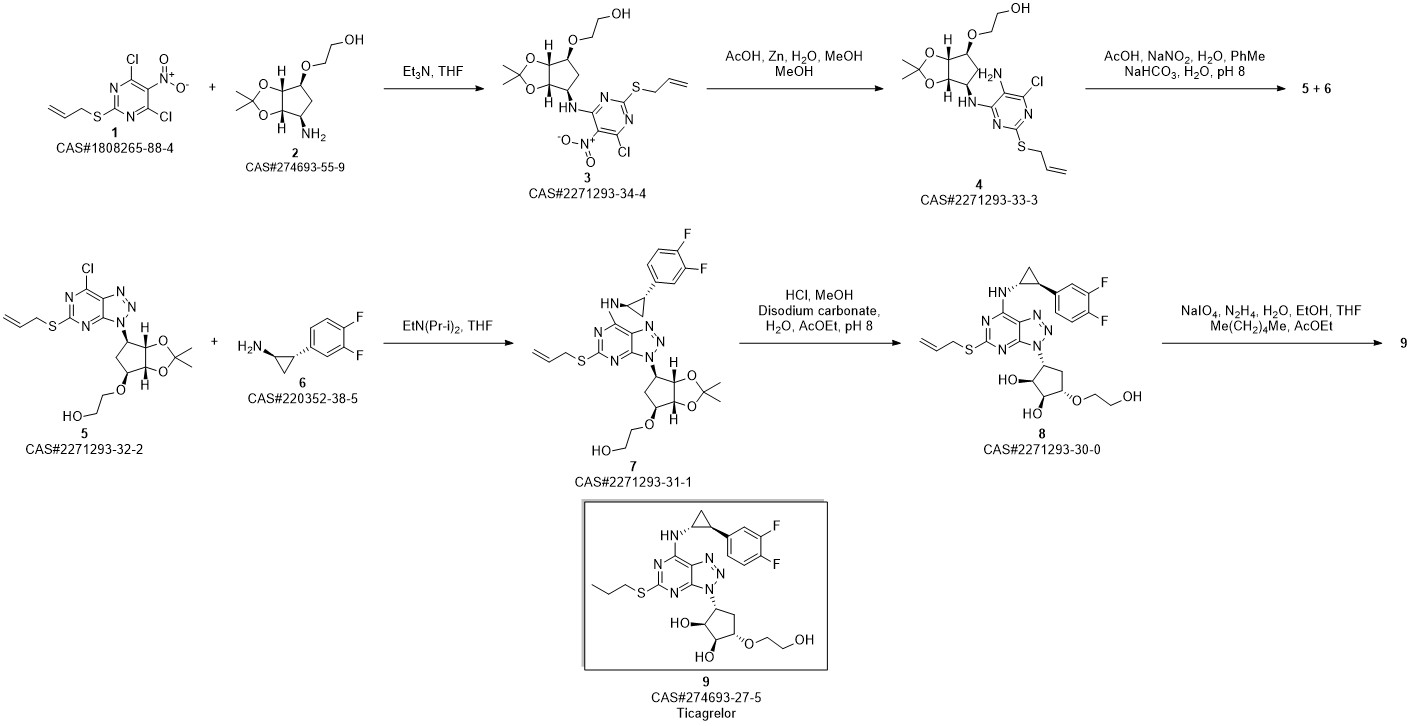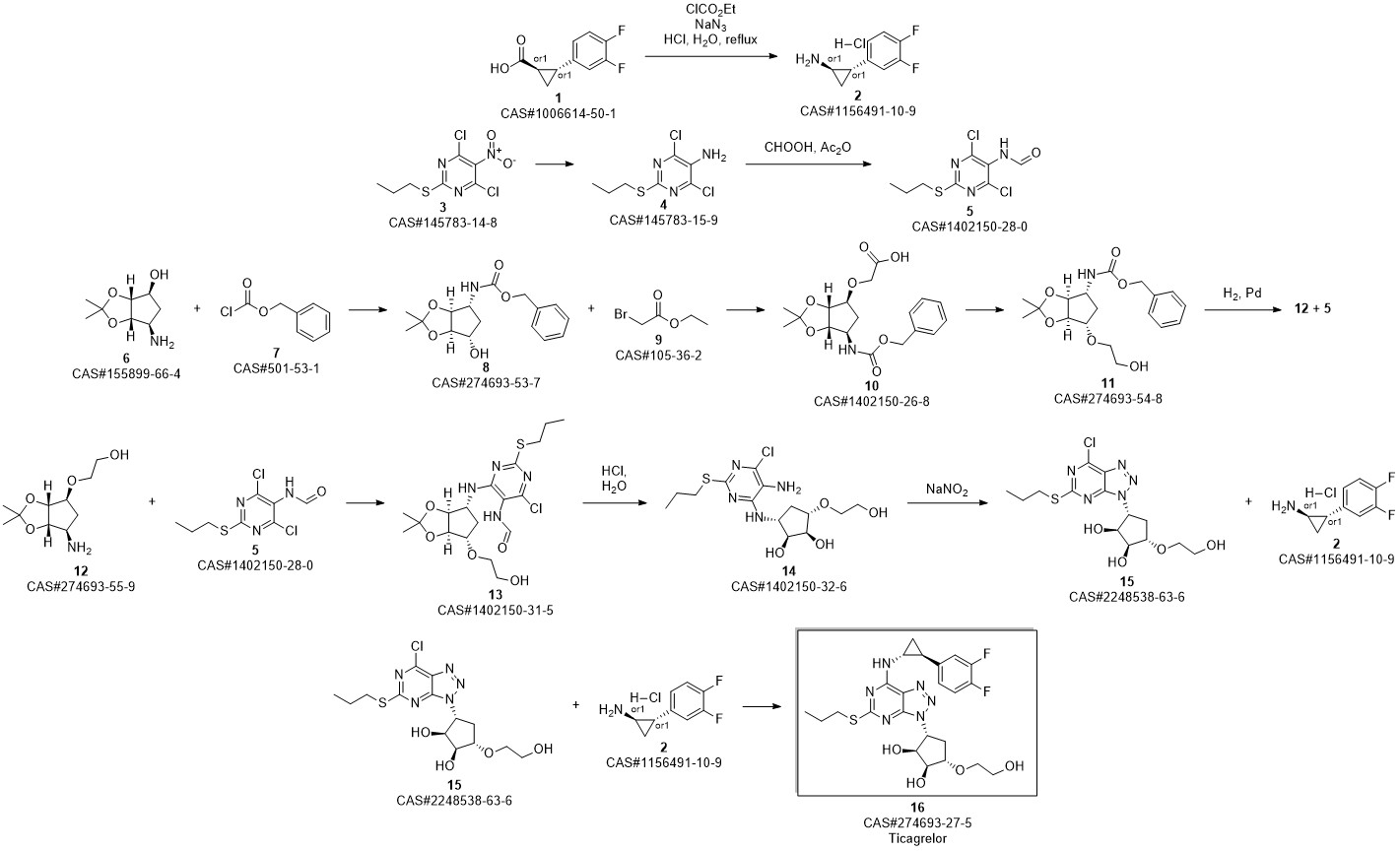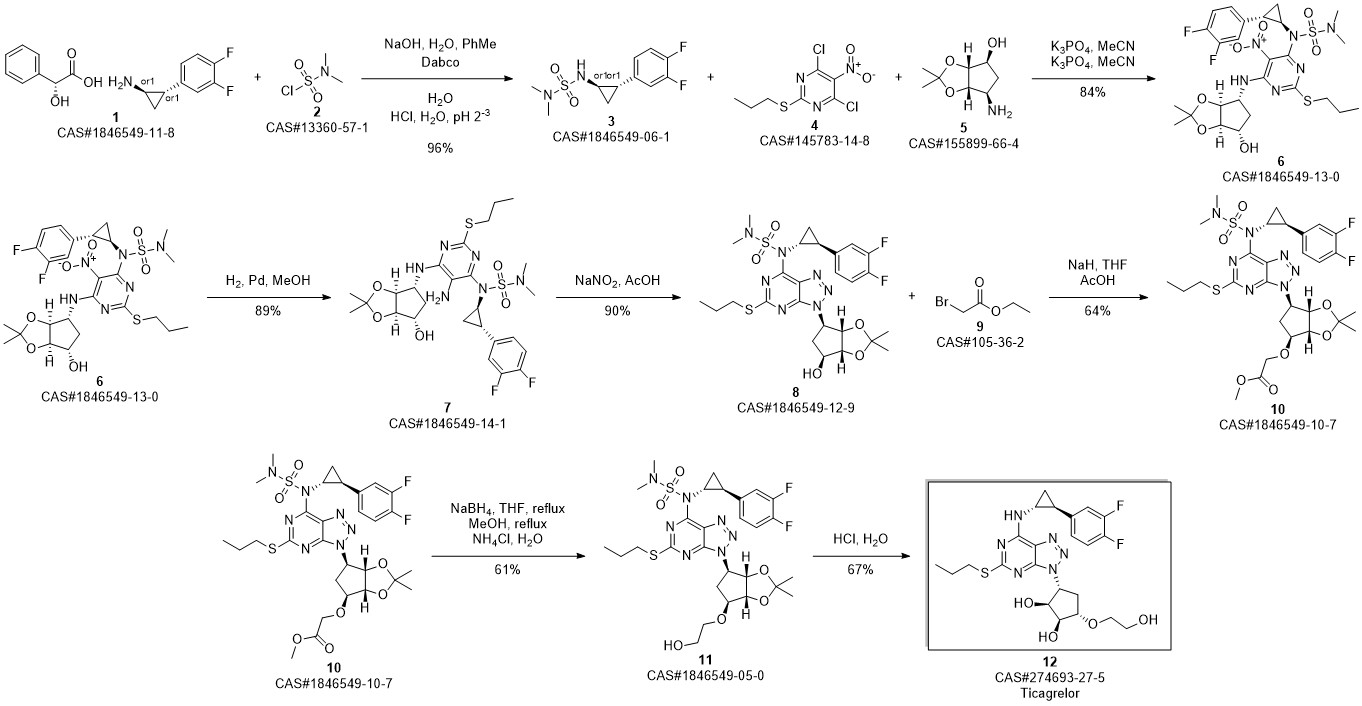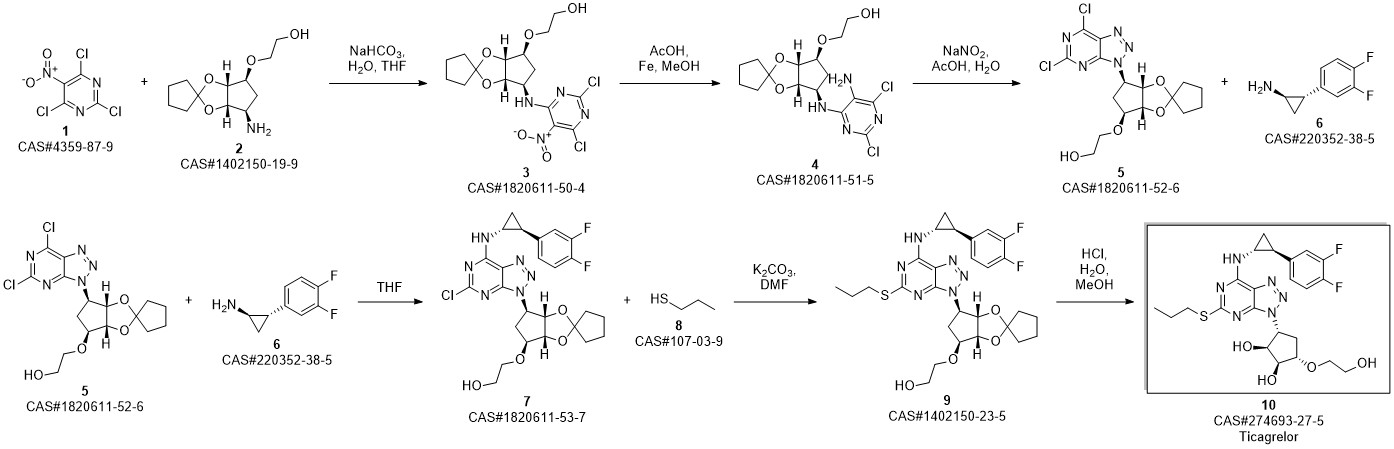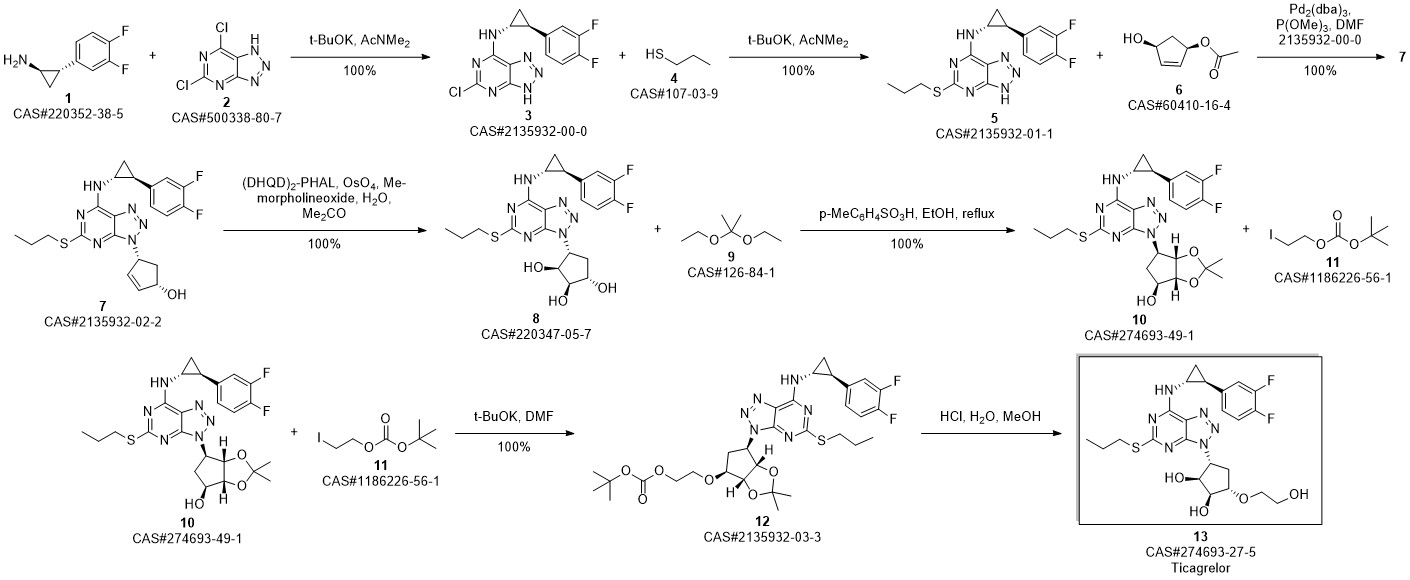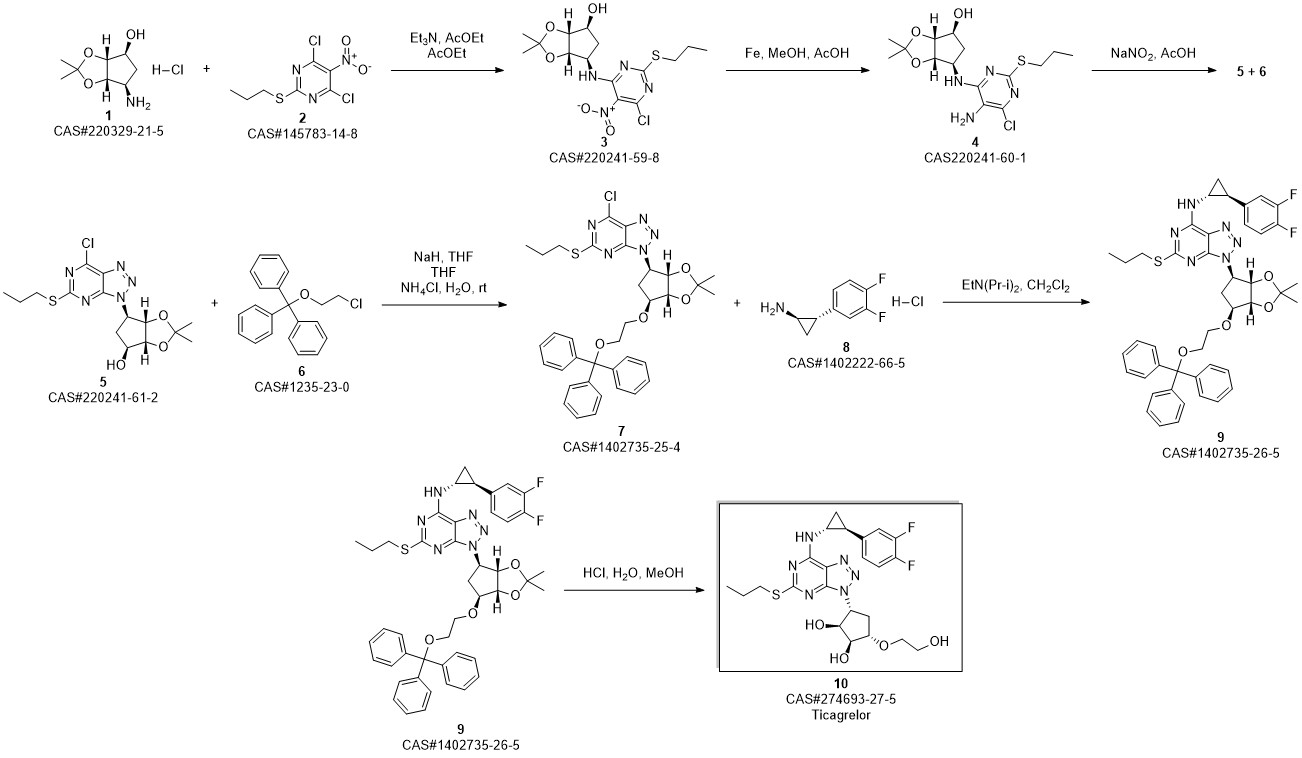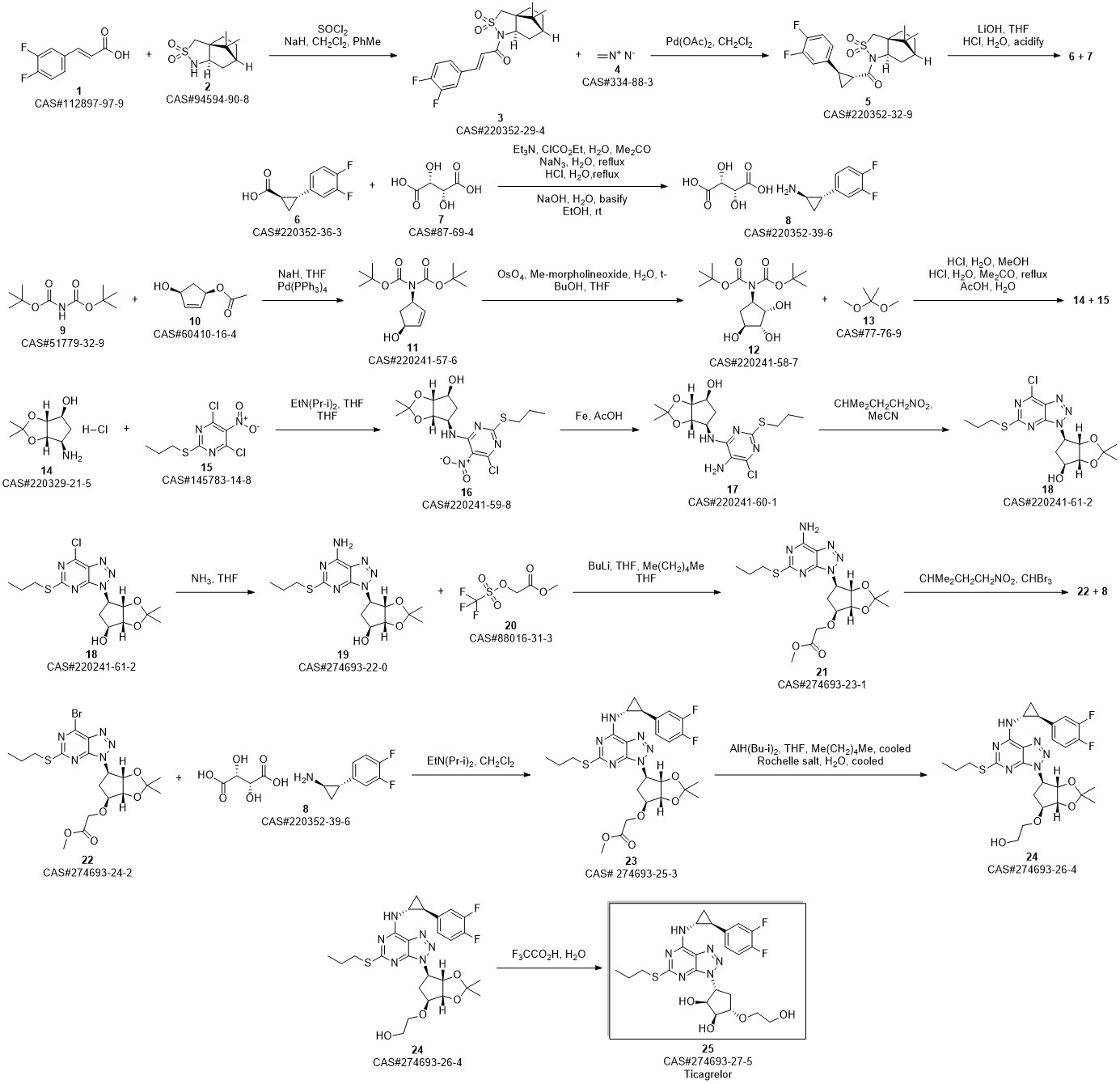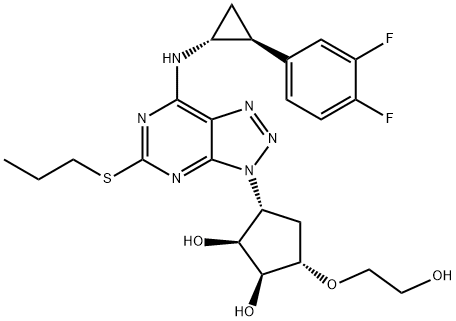
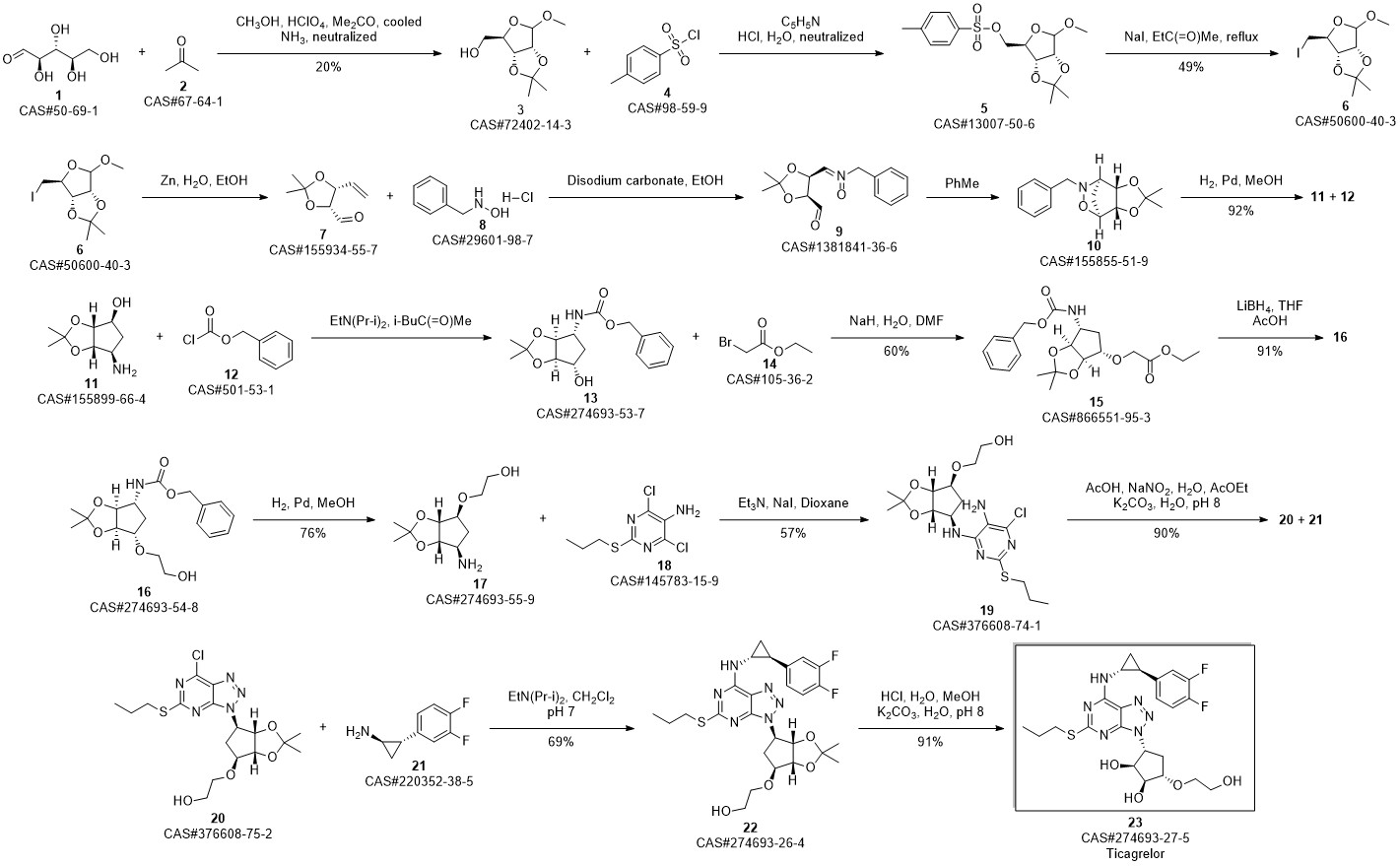
TICAGRELOR synthesis
- Product Name:TICAGRELOR
- CAS Number:274693-27-5
- Molecular formula:C23H28F2N6O4S
- Molecular Weight:522.57
Zhang, Hao; Liu, Jun; Zhang, Luyong; Kong, Lingyi; Yao, Hequan; Sun, Hongbin. Synthesis and biological evaluation of ticagrelor derivatives as novel antiplatelet agents. Bioorganic & Medicinal Chemistry Letters. Volume 22. Issue 11. Pages 3598-3602. Journal; Online Computer File. (2012).
![2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol](/CAS/20150408/GIF/274693-26-4.gif)
274693-26-4
169 suppliers
inquiry

274693-27-5
695 suppliers
$13.00/10mg
Yield:274693-27-5 97.1%
Reaction Conditions:
with hydrogenchloride in methanol at -3 - 20; for 3 h;Large scale;
Steps:
3 Example 3 Preparation of ticagrelor
Methanol (105 L) and Stage II (21 kg) were charged to the reaction kettle at room temperature,Cool to -3 ° C at -3 ° CConcentrate hydrochloric acid (107.1 kg) in batches in two hours and below,Stir for 1 hour. Methyl tert-butyl ether was charged to the reaction vessel at -3 ° C105L,207 L sodium hydroxide solution was added to adjust the pH of the reaction mass to 8.0. After the reaction mass was stirred at 10 ° C for 30 minutes,Warm to room temperature and stir for another 30 minutes.Static stratification,The organic and aqueous phases are separated,The lower aqueous phase was extracted with methyl tert-butyl ether,The organic phase was washed with saturated sodium chloride solution and then extracted with methanol.Combined organic phase,Put into activated carbon 2.10kg,Heated to 40 ° C,Stir for 30 minutes,filter,Rinse with methyl tert-butyl ether.Vacuum distillation,The resulting residue was recrystallized from ethyl acetate and cyclohexane,Centrifuge,Vacuum dried at 55 ° C for 6 hours,Ticagrelor.The yield is about 97.1%Purity is about 99.6%.
References:
CN106866682,2017,A Location in patent:Paragraph 0045; 0046; 0047; 0048
![Carbamic acid, N-[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]-N-[5-(propylthio)-3-[(3aS,4R,6S,6aR)-tetrahydro-6-(2-hydroxyethoxy)-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-yl]-3H-1,2,3-triazolo[4,5-d]pyrimidin-7-yl]-, 1,1-dimethylethyl ester](/CAS/20200515/GIF/1383715-61-4.gif)
1383715-61-4
0 suppliers
inquiry

274693-27-5
695 suppliers
$13.00/10mg
![2-[[(3aR,4S,6R,6aS)-6-(7-azanyl-5-propylsulfanyl-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,2-dimethyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]ethanol](/CAS/20180629/GIF/1354945-69-9.gif)
1354945-69-9
29 suppliers
inquiry
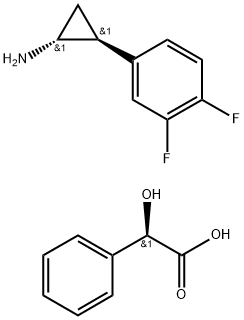
![[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]azanium:2-hydroxy-2-phenylacetate](/CAS/20180527/GIF/1444301-72-7.gif)
1444301-72-7
2 suppliers
inquiry

274693-27-5
695 suppliers
$13.00/10mg
376608-71-8
398 suppliers
$5.00/250mg
![2-[[(3aR,4S,6R,6aS)-6-(7-azanyl-5-propylsulfanyl-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-2,2-dimethyl-4,5,6,6a-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yl]oxy]ethanol](/CAS/20180629/GIF/1354945-69-9.gif)
1354945-69-9
29 suppliers
inquiry

274693-27-5
695 suppliers
$13.00/10mg
![Acetic acid, 2-[[(1S,2S,3S,4R)-4-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-2,3-dihydroxycyclopentyl]oxy]-, ethyl ester](/CAS/20200611/GIF/1402150-10-0.gif)
1402150-10-0
7 suppliers
inquiry

274693-27-5
695 suppliers
$13.00/10mg